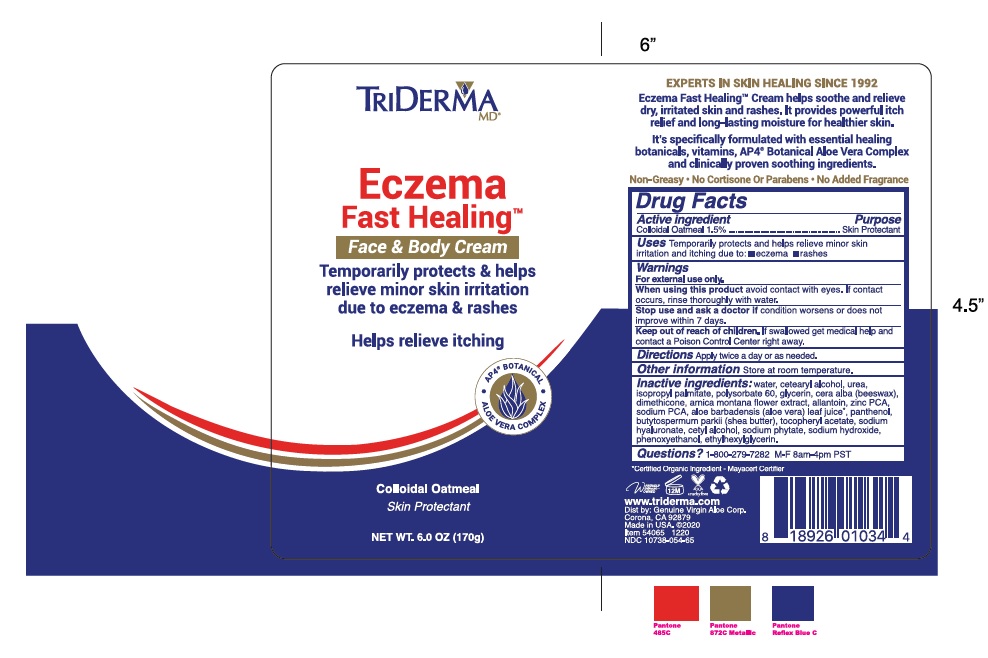
Label: TRIDERMA ECZEMA FAST HEALING FACE AND BODY- colloidal oatmeal cream
-
NDC Code(s):
10738-054-20,
10738-054-25,
10738-054-40,
10738-054-45, view more10738-054-50, 10738-054-55, 10738-054-65
- Packager: Genuine Virgin Aloe Corporation
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 31, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients: water, cetearyl alcohol, urea, isopropyl palmitate, arnica montana flower extract, glycerin, polysorbate 60, cera alba (beeswax), dimethicone, allantoin, zinc PCA, sodium PCA, aloe barbadensis leaf juice*, panthenol (pro-vitamin B5), butyrospermum parkii (shea) butter, tocopheryl acetate (vitamin E), sodium hyaluronate, cetyl alcohol, sodium phytate, sodium hydroxide, phenoxyethanol, ethylhexylglycerin.
*Certified Organic Ingredient - Mayacert Certifier - QUESTIONS
-
SPL UNCLASSIFIED SECTION
Temporarily protects & helps relieve minor skin irritation due to eczema & rashes
Helps relieve itchingAP4 BOTANICAL
ALOE VERA COMPLEX
with Colloidal Oatmeal
Maximum Strength
Healing BotanicalsEczema Fast Healing™ cream helps promote fast relief for itching and rashes due to Eczema related symptoms.
This rich cream contains essential healing botanicals, vitamins and AP4 ® Aloe Vera Complex. It provides long lasting moisture and helps
relieve itching without harmful drugs.Non-Greasy
No Added Fragrance or ParabensDist. by: Genuine Virgin Aloe Corp.,
Corona, CA 92879
•AP4® Aloe Vera Complex, a proprietary botanical blend
• Colloidal Oatmeal, helps relieve skin irritations due to Eczema and rashes
• Shea Butter, Arnica Montana Flower & Beeswax
• Vitamins B and EDERMATOLOGIST TESTED
Rooted from a medical heritage, TriDerma MD ® was founded after one woman’s struggle to find safe and effective skin healing.
At TriDerma MD ®, we strive to create the perfect healing formulations for specific skin irritations. Our products are made using only
the finest quality, most effective ingredients. We are passionate in our pursuit of fast healing, natural botanicals that do not cause
harmful side effects.Quality you can feel,
results you can see. ® - Packaging
-
INGREDIENTS AND APPEARANCE
TRIDERMA ECZEMA FAST HEALING FACE AND BODY
colloidal oatmeal creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10738-054 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OATMEAL (UNII: 8PI54V663Y) (OATMEAL - UNII:8PI54V663Y) OATMEAL 1.5 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) UREA (UNII: 8W8T17847W) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 60 (UNII: CAL22UVI4M) WHITE WAX (UNII: 7G1J5DA97F) DIMETHICONE (UNII: 92RU3N3Y1O) ALLANTOIN (UNII: 344S277G0Z) ZINC PIDOLATE (UNII: C32PQ86DH4) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) ALOE VERA LEAF (UNII: ZY81Z83H0X) PANTHENOL (UNII: WV9CM0O67Z) SHEA BUTTER (UNII: K49155WL9Y) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CETYL ALCOHOL (UNII: 936JST6JCN) PHYTATE SODIUM (UNII: 88496G1ERL) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10738-054-50 1 in 1 CARTON 10/01/2019 1 NDC:10738-054-55 14 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:10738-054-20 1 in 1 CARTON 10/01/2019 2 NDC:10738-054-25 62 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:10738-054-40 1 in 1 CARTON 10/01/2019 3 NDC:10738-054-45 119 g in 1 TUBE; Type 0: Not a Combination Product 4 NDC:10738-054-65 170 g in 1 TUBE; Type 0: Not a Combination Product 10/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 10/01/2019 Labeler - Genuine Virgin Aloe Corporation (961374147) Establishment Name Address ID/FEI Business Operations Genuine Virgin Aloe Corporation 961374147 manufacture(10738-054)