Label: VIVIER SHEER SPF 45- zinc oxide, octocrylene, octinoxate, octisalate lotion
- NDC Code(s): 67226-2852-0, 67226-2852-1
- Packager: Vivier Pharma, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
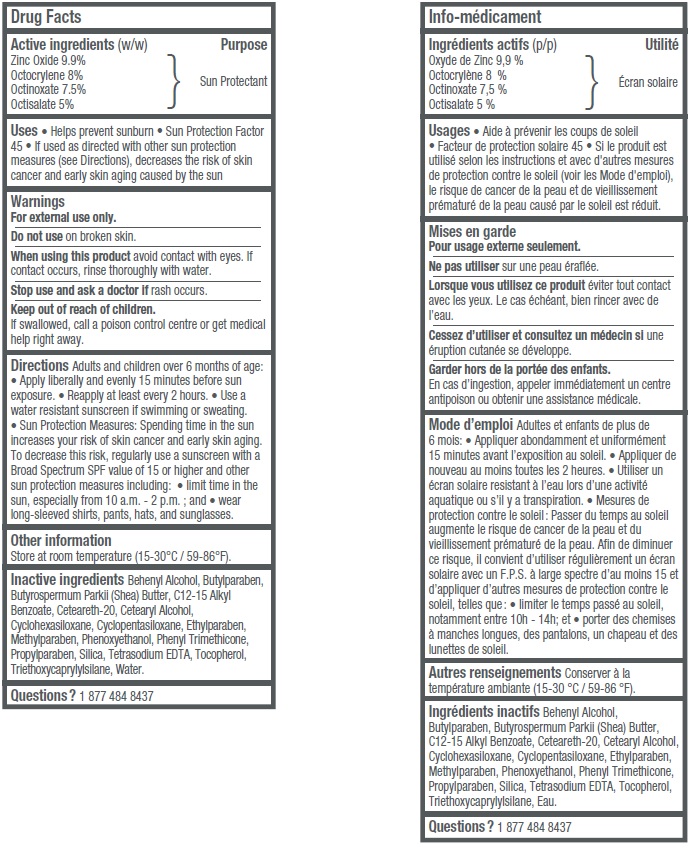
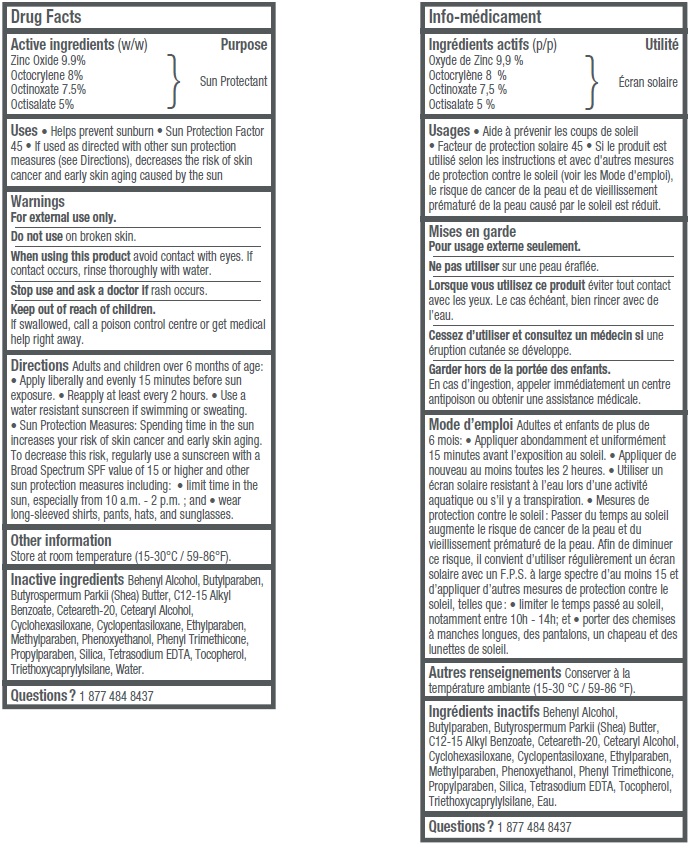
- Active ingredients (w/w)
- Uses
- Warnings
-
Directions
Adults and children over 6 months of age: • Apply liberally and evenly 15 minutes before sun exposure • Reapply at least every 2 hours • Use a water resistant sunscreen if swimming or sweating. • Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m.– 2 p.m.; and • wear long-sleeved shirts, pants, hats, and sunglasses.
- Other information
-
Inactive ingredients
Behenyl Alcohol, Butylparaben, Butyrospermum Parkii (Shea) Butter, C12-15 Alkyl Benzoate, Ceteareth-20, Cetearyl Alcohol, Cyclohexasiloxane, Cyclopentasiloxane, Ethylparaben, Methylparaben, Phenoxyethanol, Phenyl Trimethicone, Propylparaben, Silica, Tetrasodium EDTA, Tocopherol, Triethoxycaprylylsilane, Water.
- Questions?
- Package Labeling:90ml
- Package Labeling:250ml
-
INGREDIENTS AND APPEARANCE
VIVIER SHEER SPF 45
zinc oxide, octocrylene, octinoxate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67226-2852 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 99 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 80 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength DOCOSANOL (UNII: 9G1OE216XY) BUTYLPARABEN (UNII: 3QPI1U3FV8) SHEA BUTTER (UNII: K49155WL9Y) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ETHYLPARABEN (UNII: 14255EXE39) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) PROPYLPARABEN (UNII: Z8IX2SC1OH) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) EDETATE SODIUM (UNII: MP1J8420LU) TOCOPHEROL (UNII: R0ZB2556P8) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67226-2852-0 1 in 1 CARTON 01/05/2023 1 90 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:67226-2852-1 1 in 1 CARTON 01/05/2023 2 250 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/05/2023 Labeler - Vivier Pharma, Inc. (250996550)