Label: SPF 30 DAILY SUNSCREEN- zinc oxide cream
- NDC Code(s): 81136-001-01
- Packager: Brand Evangelists for Beauty Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
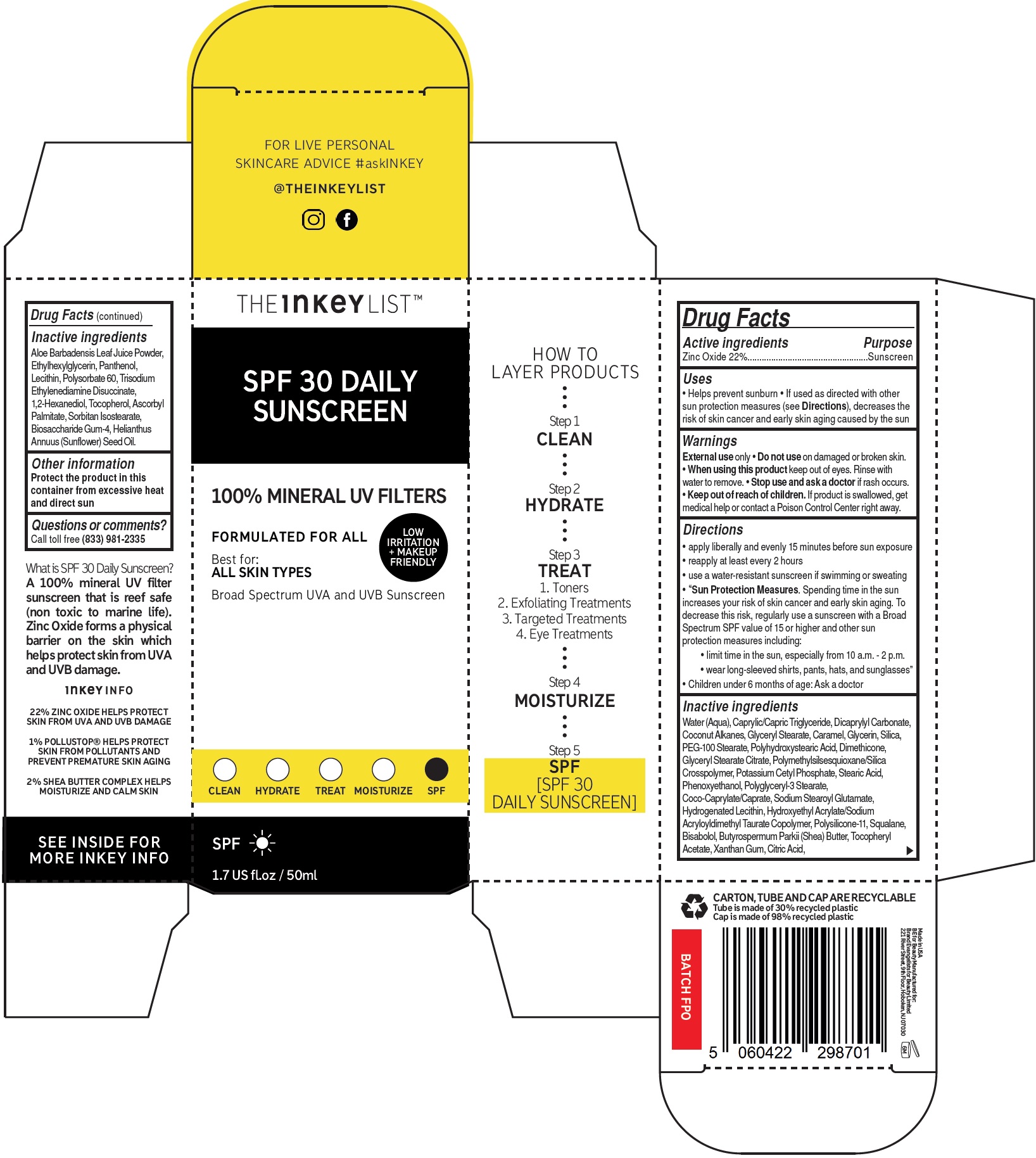
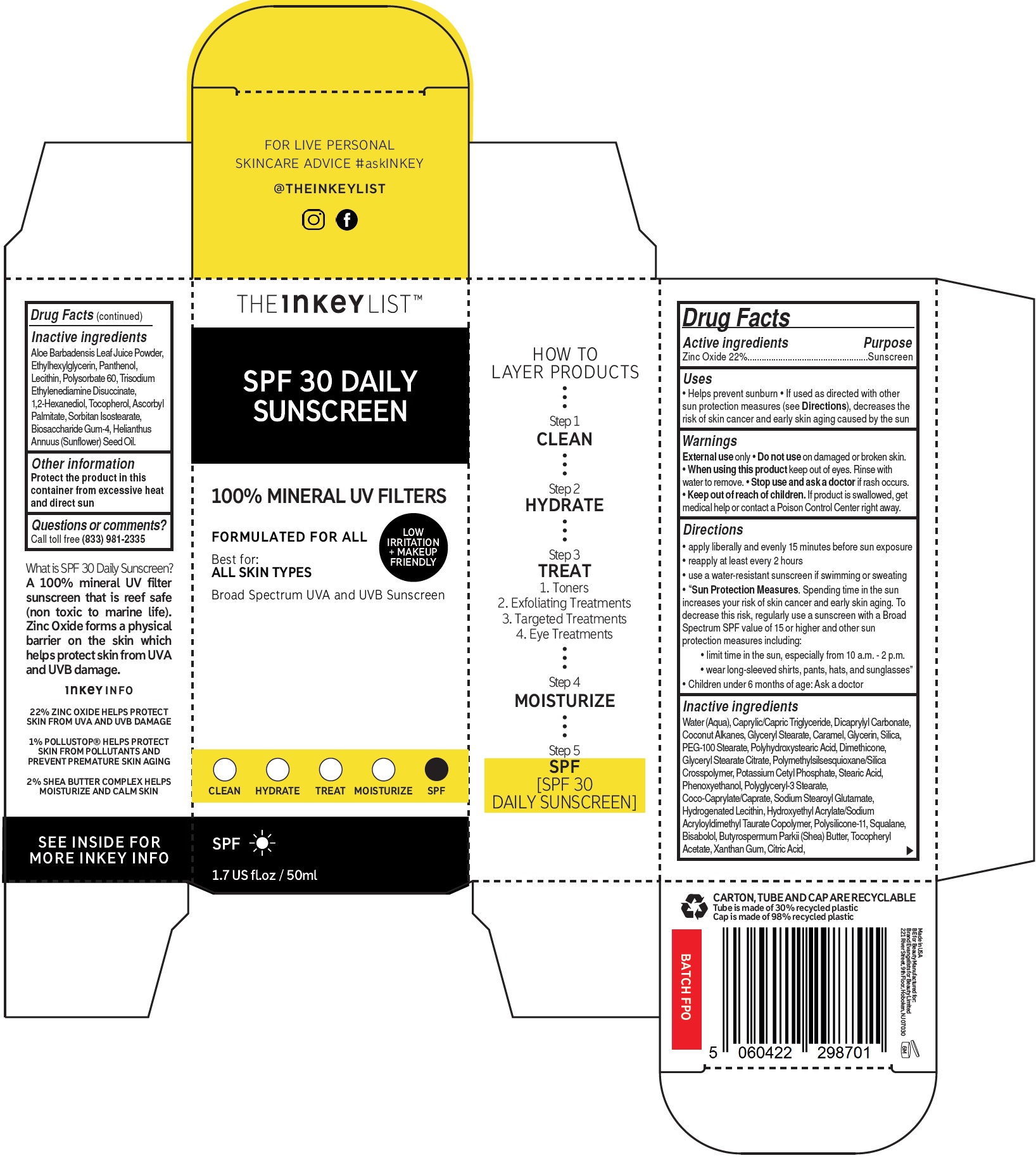
- Drug Facts
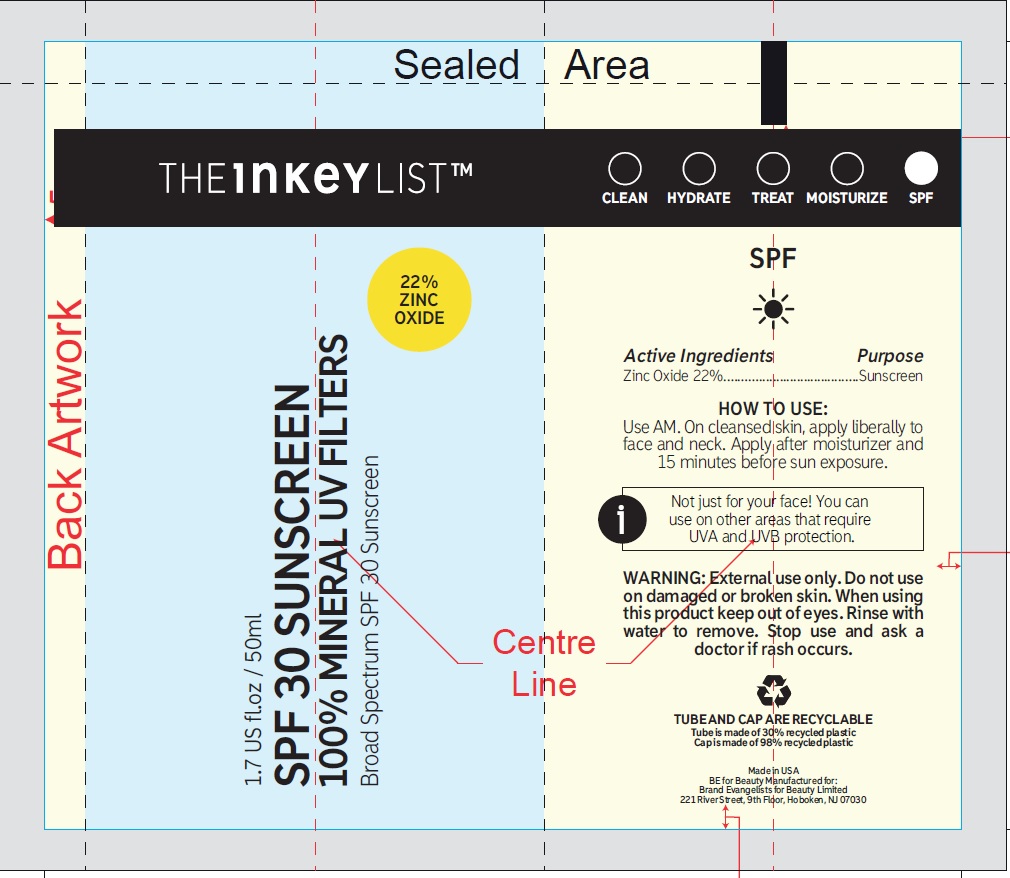
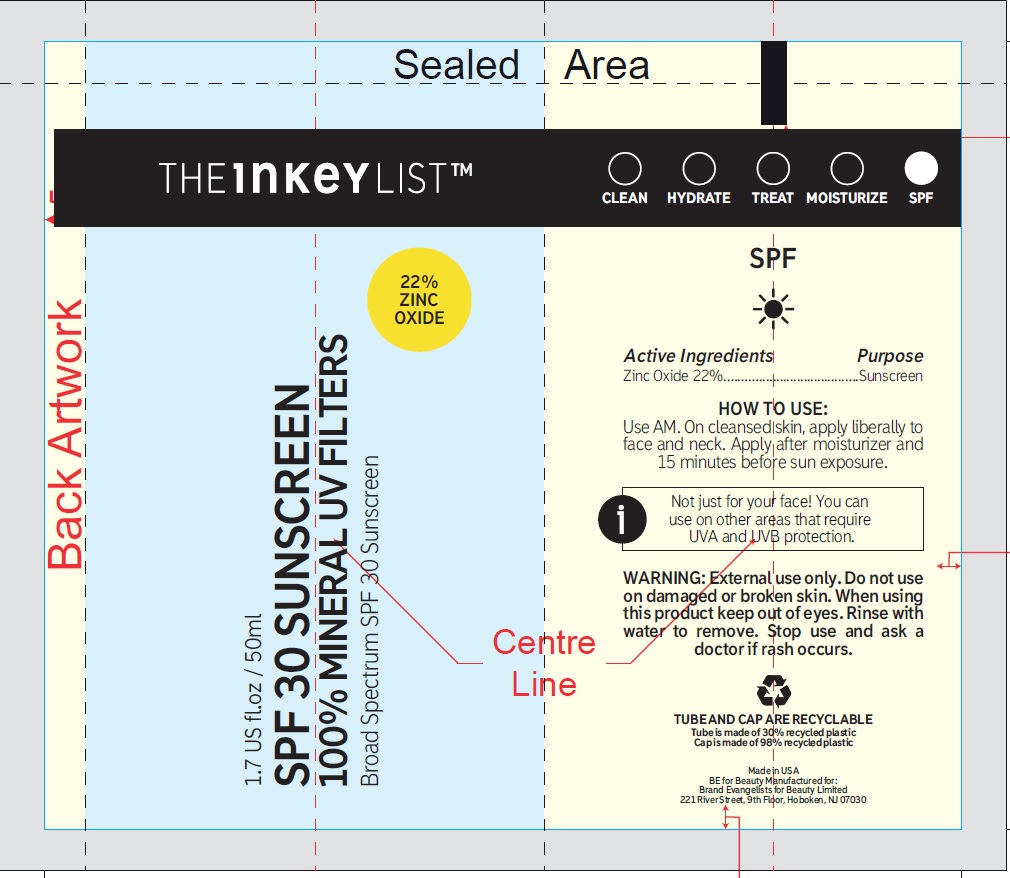
- Active ingredients
- Uses
- Warnings
-
Directions
- apply liberally and evenly 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water-resistant sunscreen if swimming or sweating
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m. - 2 p.m. • wear long-sleeved shirts, pants, hats, and sunglasses”
“Sun Protection Measures.
- Children under 6 months of age: Ask a doctor
-
Inactive ingredients
Water (Aqua), Caprylic/Capric Triglyceride, Dicaprylyl Carbonate, Coconut Alkanes, Glyceryl Stearate, Caramel, Glycerin, Silica, PEG-100 Stearate, Polyhydroxystearic Acid, Dimethicone, Glyceryl Stearate Citrate, Polymethylsilsesquioxane/Silica Crosspolymer, Potassium Cetyl Phosphate, Stearic Acid, Phenoxyethanol, Polyglyceryl-3 Stearate, Coco-Caprylate/Caprate, Sodium Stearoyl Glutamate, Hydrogenated Lecithin, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Polysilicone-11, Squalane, Bisabolol, Butyrospermum Parkii (Shea) Butter, Tocopheryl Acetate, Xanthan Gum, Citric Acid,Aloe Barbadensis Leaf Juice Powder, Ethylhexylglycerin, Panthenol, Lecithin, Polysorbate 60, Trisodium Ethylenediamine Disuccinate, 1,2-Hexanediol, Tocopherol, Ascorbyl Palmitate, Sorbitan Isostearate, Biosaccharide Gum-4, Helianthus Annuus (Sunflower) Seed Oil.
- Other information
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SPF 30 DAILY SUNSCREEN
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81136-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 220 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) COCONUT ALKANES (UNII: 1E5KJY107T) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CARAMEL (UNII: T9D99G2B1R) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PEG-100 STEARATE (UNII: YD01N1999R) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE CITRATE (UNII: WH8T92A065) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) STEARIC ACID (UNII: 4ELV7Z65AP) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-3 STEARATE (UNII: 8FDA8C98S3) COCOYL CAPRYLOCAPRATE (UNII: 8D9H4QU99H) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SQUALANE (UNII: GW89575KF9) LEVOMENOL (UNII: 24WE03BX2T) SHEA BUTTER (UNII: K49155WL9Y) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) XANTHAN GUM (UNII: TTV12P4NEE) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ALOE VERA LEAF (UNII: ZY81Z83H0X) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PANTHENOL (UNII: WV9CM0O67Z) POLYSORBATE 60 (UNII: CAL22UVI4M) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) TOCOPHEROL (UNII: R0ZB2556P8) ASCORBYL PALMITATE (UNII: QN83US2B0N) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) SUNFLOWER OIL (UNII: 3W1JG795YI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81136-001-01 1 in 1 CARTON 04/19/2021 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/19/2021 Labeler - Brand Evangelists for Beauty Ltd. (222990724)