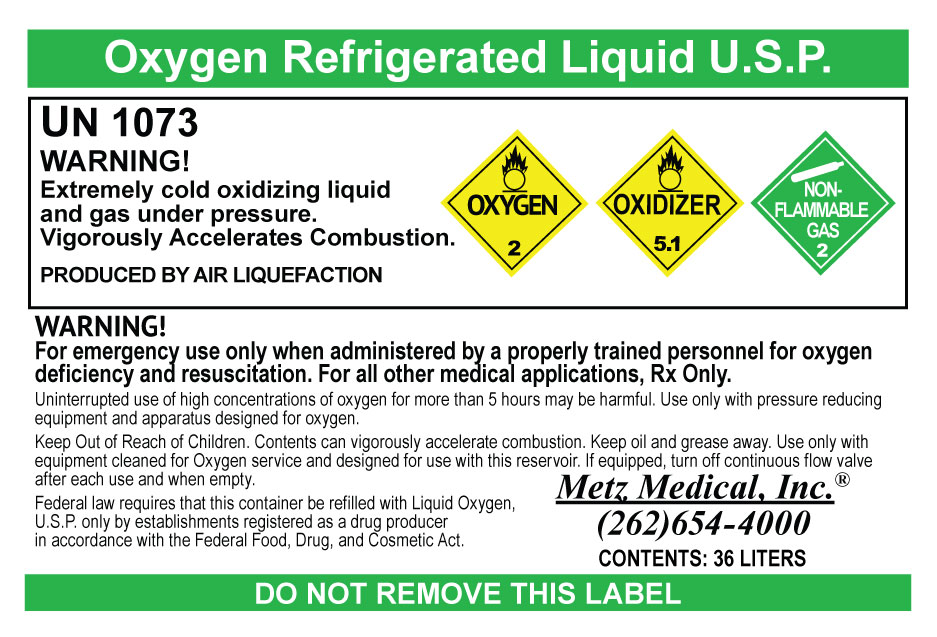
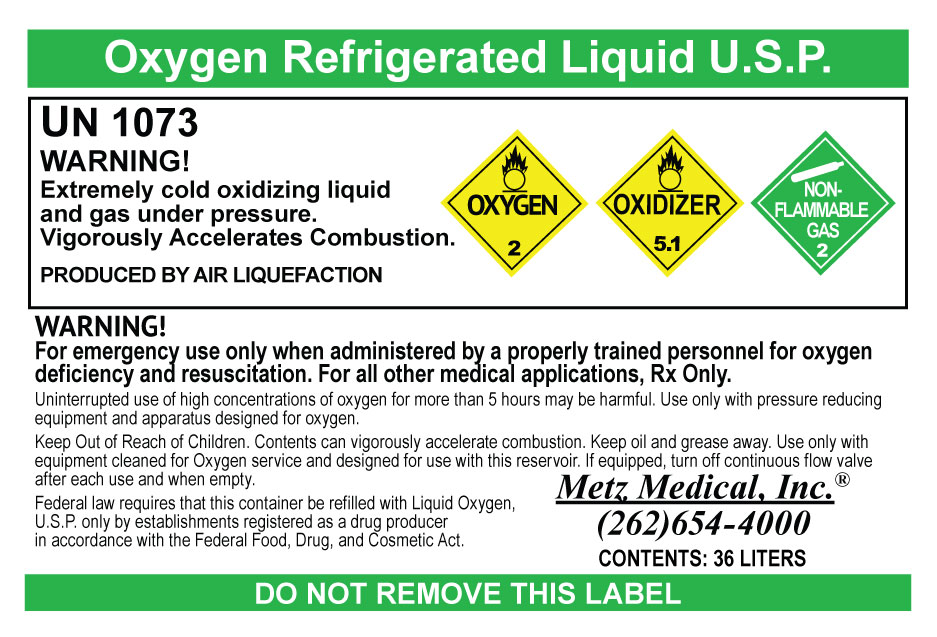
Label: LIQUID OXYGEN- oxygen gas
-
Contains inactivated NDC Code(s)
NDC Code(s): 66767-998-01, 66767-998-02, 66767-998-03 - Packager: Metz Medical, Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 3, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIQUID OXYGEN
oxygen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66767-998 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYGEN (UNII: S88TT14065) (OXYGEN - UNII:S88TT14065) OXYGEN 99 L in 100 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66767-998-01 8600 L in 1 DEWAR; Type 0: Not a Combination Product 12/03/2015 2 NDC:66767-998-02 18060 L in 1 DEWAR; Type 0: Not a Combination Product 12/03/2015 3 NDC:66767-998-03 30960 L in 1 DEWAR; Type 0: Not a Combination Product 12/03/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205712 12/03/2015 Labeler - Metz Medical, Inc (956306062) Establishment Name Address ID/FEI Business Operations Metz Medical, Inc 956306062 repack(66767-998) Establishment Name Address ID/FEI Business Operations Air Liquide Industrial U.S. LP 831995829 manufacture(66767-998)