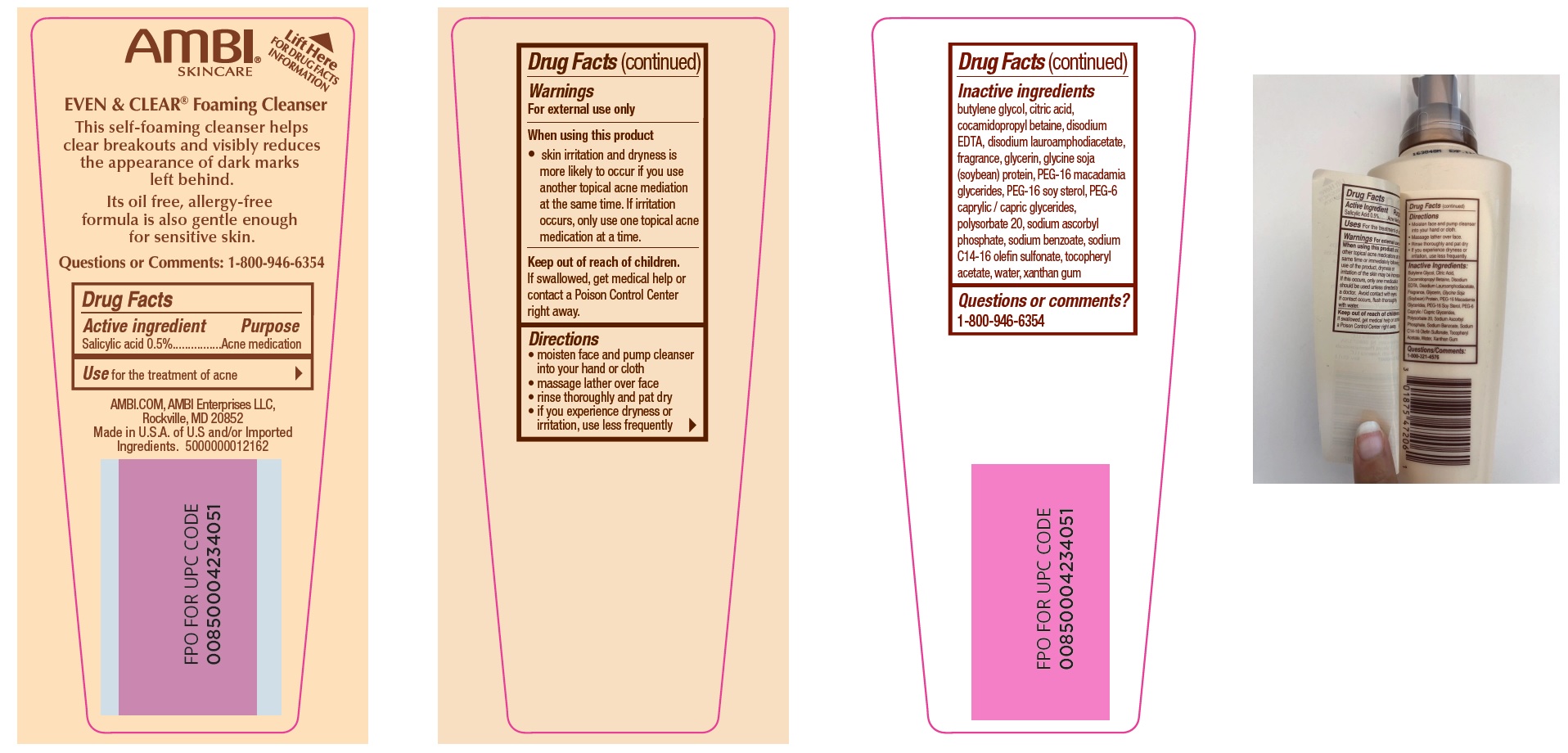
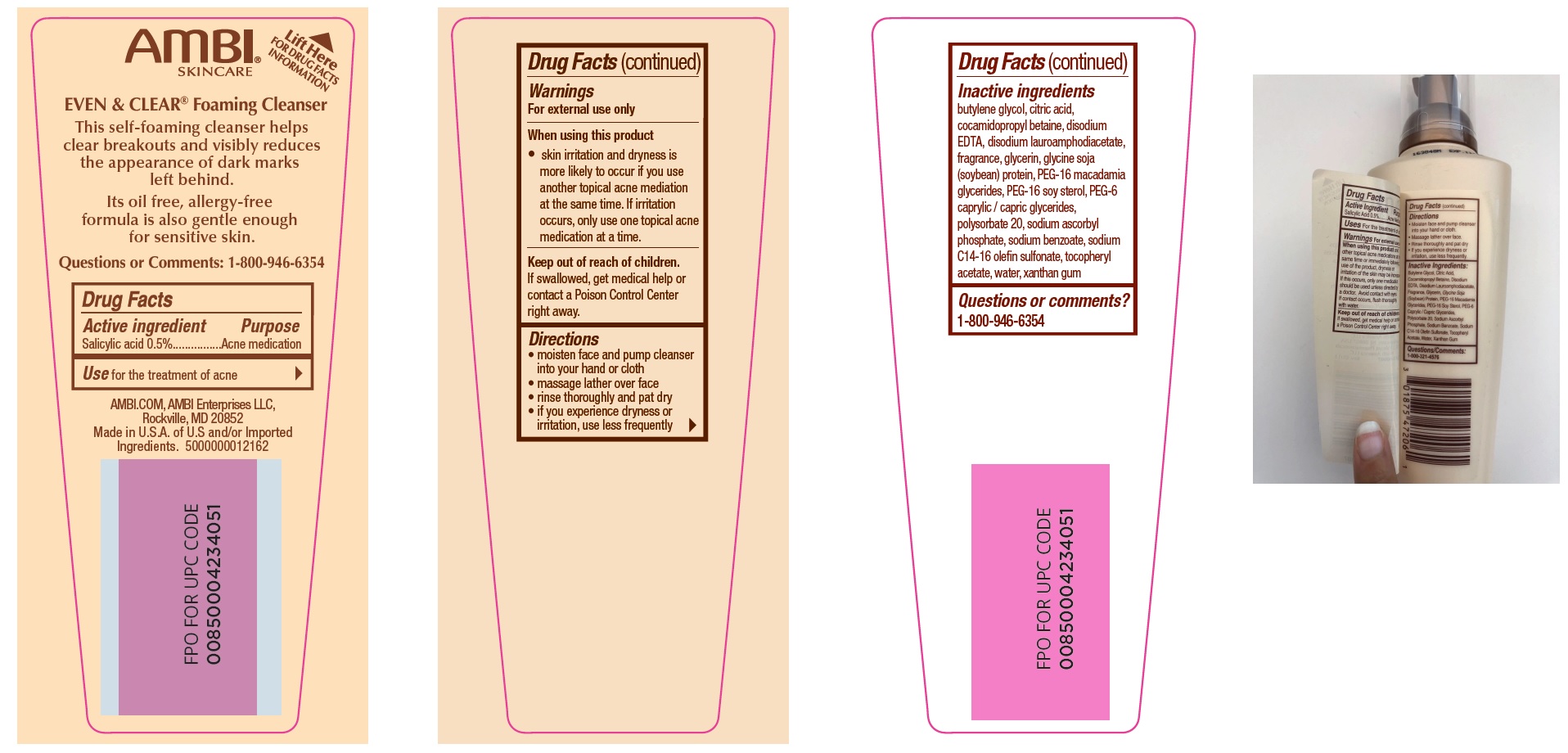
Label: EVEN AND CLEAR CLEANSER- salicylic acid liquid
- NDC Code(s): 73453-003-01
- Packager: Ambi Enterprises LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Use
- Warnings
- Directions
-
Inactive ingredients
butylene glycol, citric acid, cocamidopropyl betaine, disodium EDTA, disodium lauroamphodiacetate, fragrance, glycerin, glycine soja (soybean) protein, PEG-16 macadamia glycerides, PEG-16 soy sterol, PEG-6 caprylic / capric glycerides, polysorbate 20, sodium ascorbyl phosphate, sodium benzoate, sodium C14-16 olefin sulfonate, tocopheryl acetate, water, xanthan gum
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
EVEN AND CLEAR CLEANSER
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73453-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength XANTHAN GUM (UNII: TTV12P4NEE) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) DISODIUM LAUROAMPHODIACETATE (UNII: R4HKX6I64R) GLYCERIN (UNII: PDC6A3C0OX) SOYBEAN (UNII: L7HT8F1ZOD) PEG-16 MACADAMIA GLYCERIDES (UNII: 2OTC93KMHU) PEG-16 SOY STEROL (UNII: 9J0FEN30IT) PEG-6 CAPRYLIC/CAPRIC GLYCERIDES (UNII: GO50W2HWO8) POLYSORBATE 20 (UNII: 7T1F30V5YH) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73453-003-01 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/15/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 06/15/2014 Labeler - Ambi Enterprises LLC (117015229)