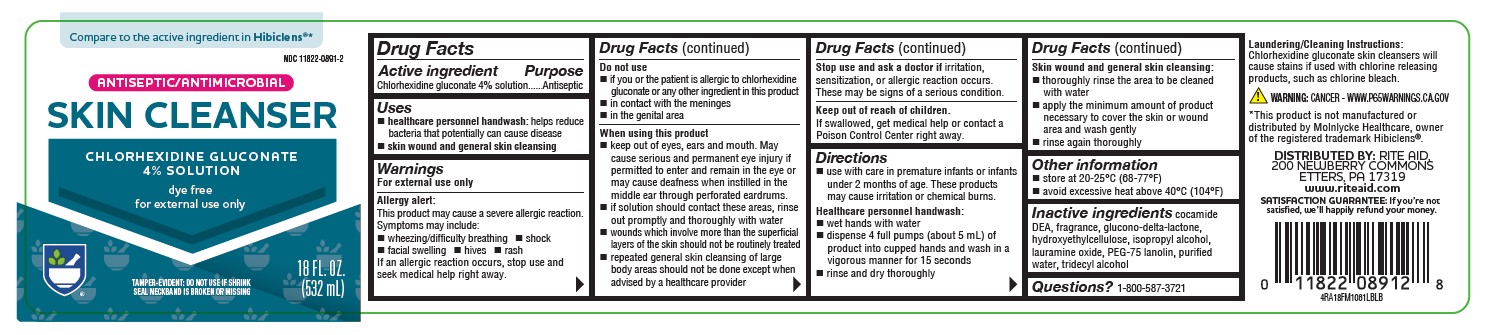
Label: ANTISEPTIC SKIN CLEANSER- chlorhexidine gluconate 4% solution
- NDC Code(s): 11822-0891-2
- Packager: Rite Aid
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use
-
When using this product
- keep out of eyes, ears and mouth. May cause serious and permanent eye injury if permitted to enter and remain in the ete or may cause deafness when instilled in the middle ear through perforated eardurms.
- if solution should contact these areas, rinse out promptly and thoroughly with water
- wounds which involve more than the superficial layers of the skin should not be routinely treated
- repeated general skin cleansing of large body areas should not be done except when advised by a healthcare provider
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- use with care in premature infants or infants under two months of age. These products may cause irritation or chemical burns.
Healthcare personnel handwash:
- wet hands with water
- dispense 4 full pumps (6 grams) of product into cupped hands and wash in a vigrous manner for 15 seconds
- rinse and dry thoroughly
Skin wound and general skin cleansing:
- thoroughly rinse the area to be cleaned with water
- apply the minimum amount of product necessary to cover the skin or wound area and wash gently
- rinse again thoroughly
- Other information
- Inactive ingredients
- Questions or comments?
- OTHER SAFETY INFORMATION
-
PRINCIPAL DISPLAY PANEL
Compare to the active ingredient in Hibiclens®*
NDC 11822-0891-2
ANTISEPTIC/ANTIMICROBIAL
SKIN CLEANSER
CHLORHEXIDINE GLUCONATE
4% SOLUTION
dye free
for external use only
TAMPER-EVIDENT: DO NOT USE IF SHRINK SEAL NECKBAND IS BROKEN OR MISSING
18 FL OZ (532 mL)
4RA18FM1061LBLB
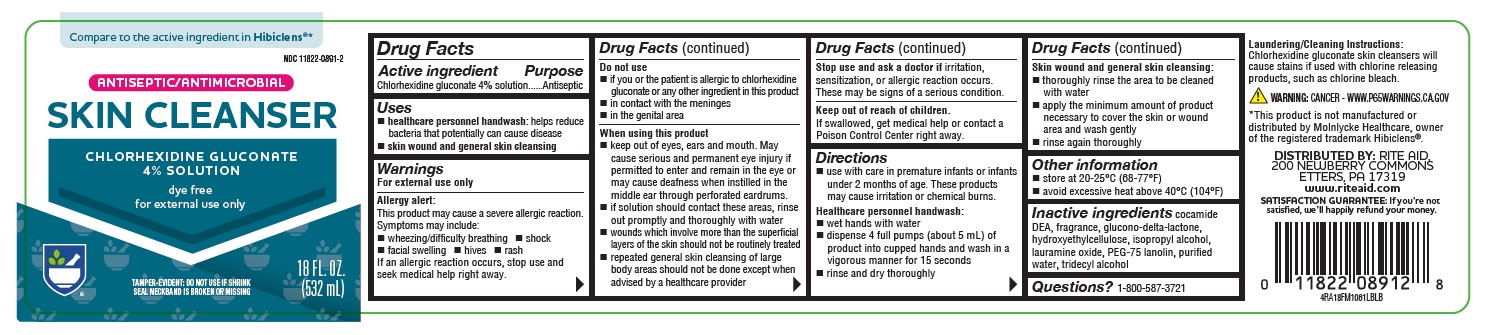
*This product is not manufactured or distributed by Molnlycke Healthcare, owner of the registered trademark Hibiclens®.
DISTRIBUTED BY: RITE AID, 200 NEWBERRY COMMONS
ETTERS, PA 17319
www.riteaid.com
SATISFACTION GUARANTEE: If you’re not satisfied, we’ll happily refund your money. -
INGREDIENTS AND APPEARANCE
ANTISEPTIC SKIN CLEANSER
chlorhexidine gluconate 4% solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11822-0891 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 4 g in 100 mL Inactive Ingredients Ingredient Name Strength DIHYDROXYETHYL LAURAMINE OXIDE (UNII: JD265DM4U6) PEG-75 LANOLIN (UNII: 09179OX7TB) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) WATER (UNII: 059QF0KO0R) TRIDECYL ALCOHOL (UNII: 8I9428H868) GLUCONOLACTONE (UNII: WQ29KQ9POT) ISOPROPYL ALCOHOL (UNII: ND2M416302) COCO DIETHANOLAMIDE (UNII: 92005F972D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11822-0891-2 532 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 04/14/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019125 04/14/2021 Labeler - Rite Aid (014578892) Registrant - Xttrium Laboratories, Inc. (007470579) Establishment Name Address ID/FEI Business Operations Xttrium Laboratories, Inc 007470579 api manufacture(11822-0891)