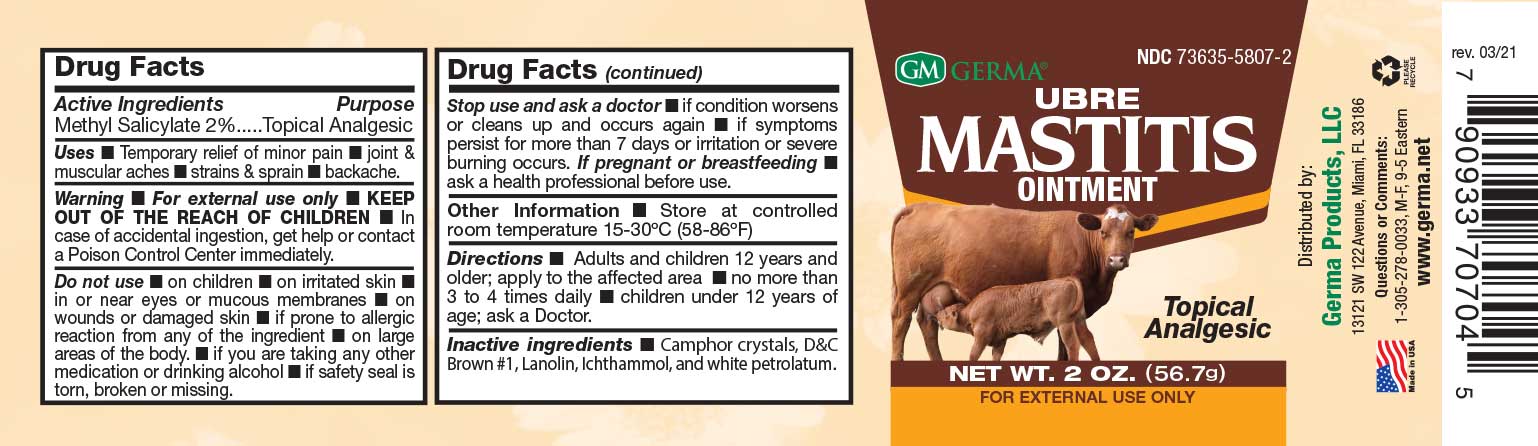
Label: GERMA UBRE MASTITIS- methyl salicylate 2% ointment
- NDC Code(s): 73635-5807-2
- Packager: Germa Products, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Uses
- INDICATIONS & USAGE
- Purpose
- Warnings
-
Warnings
KEEP OUT OF THE REACH OF CHILDREN
In case of accidental ingestion, get help or contact a Poison Control Center immediately.
Do not use • on children • on irritated skin • in or near eyes or mucous membranes • on wounds or damaged skin n if prone to allergic reaction from any of the ingredient n on large areas of the body. • if you are taking any other medication or drinking alcohol • if safety seal is torn, broken or missing. - PREGNANCY OR BREAST FEEDING
- ASK DOCTOR
- Other Information
- Directions
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GERMA UBRE MASTITIS
methyl salicylate 2% ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73635-5807 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WHITE PETROLATUM (UNII: B6E5W8RQJ4) D&C BROWN NO. 1 (UNII: 8796B4I6HE) LANOLIN OIL (UNII: OVV5IIJ58F) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) ICHTHAMMOL (UNII: NQ14646378) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73635-5807-2 56.69 mL in 1 JAR; Type 0: Not a Combination Product 03/29/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M014 03/29/2019 Labeler - Germa Products, LLC (116626935)