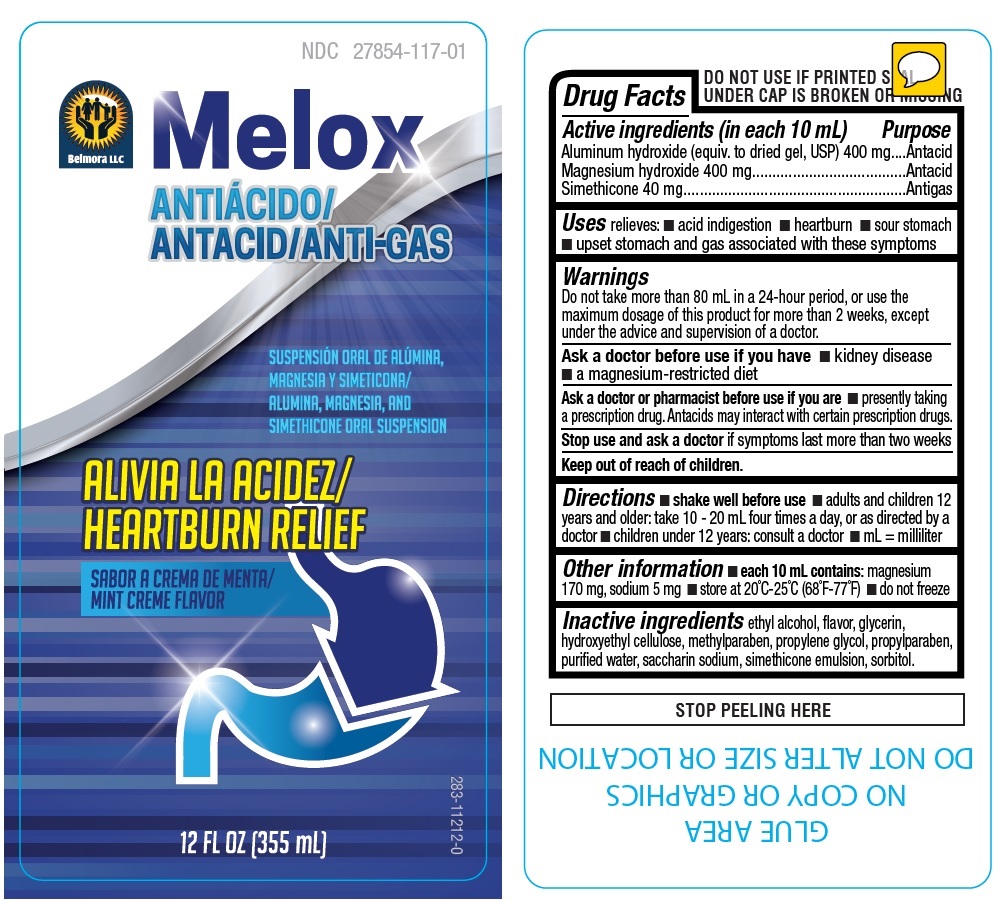
Label: MELOX- aluminum hydroxide, magnesium hydroxide, simethicone liquid
- NDC Code(s): 27854-117-01
- Packager: Belmora LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients (in each 10 mL)
- Purpose
- Uses
-
Warnings
Do not take more than 80 mL in a 24-hour period, or use the maximum dosage of this product for more than 2 weeks, except under the advice and supervision of a doctor.
Ask a doctor before use if you have • kidney disease • a magnesium-restricted diet
Ask a doctor or pharmacist before use if you are • presently taking a prescription drug. Antacids may interact with certain prescription drugs.
Stop use and ask a doctor if symptoms last more than two weeks
- Directions
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
MELOX
aluminum hydroxide, magnesium hydroxide, simethicone liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:27854-117 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 400 mg in 10 mL MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM HYDROXIDE 400 mg in 10 mL DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 40 mg in 10 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL (UNII: 506T60A25R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:27854-117-01 355 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 05/01/2023 Labeler - Belmora LLC (112753244)