Label: COMPONENT E-C WITH TYLAN- progesterone, estradiol benzoate, tylosin tartrate implant
- NDC Code(s): 58198-0507-2
- Packager: Elanco US Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
Elanco™
Component™
E-C with Tylan™
(progesterone and estradiol benzoate and tylosin tartrate implant)
BEEF CALVES 45 DAYS OF AGE AND OLDER
AND WEIGHING UP TO 400 LBS
Each implant consists of 100 mg progesterone USP and 10 mg estradiol benzoate and 29 mg tylosin tartrate
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
IMPLANTS FOR BEEF CALVES 45 DAYS OF AGE AND OLDER AND WEIGHING UP TO 400 LBS
Use with a Component Implanter
-
DESCRIPTION:
Each cartridge belt holds 20 doses of Component E-C with Tylan (progesterone and estradiol benzoate and tylosin tartrate implant) Implants. Each dose of 5 pellets consists of 4 pellets containing a total of 100 mg progesterone USP and 10 mg estradiol benzoate plus 1 pellet containing 29 mg tylosin tartrate as a local antibacterial.
-
INDICATIONS FOR USE:
For increased rate of weight gain in beef calves 45 days of age and older and weighing up to 400 lbs.
This implant is not approved for repeated implantation (reimplantation) with this or any other cattle ear implant as safety and effectiveness has not been evaluated.
Do not use in calves less than 45 days of age or veal calves because effectiveness and safety have not been evaluated.
Do not use in animals intended for subsequent breeding, or in dairy cows.
-
DIRECTIONS:
Administer 1 implant - the entire contents of one cartridge cell (5 pellets) - subcutaneously in the back of the middle third of the ear. Study and carefully follow at all times the "IMPLANTING INSTRUCTIONS" presented below, avoiding short cuts. Skin infection can be avoided by properly preparing implant site and implanter. During fly season use fly repellent on implant site. One designated team member should always do the implanting. Cleanliness of hands and instruments is important at all times.
Withdrawal Periods and Residue Warnings
No withdrawal period is required when used according to labeling.
Do not use in beef calves less than 45 days of age, dairy calves, and veal calves. Do not use in bull calves intended for reproduction. A withdrawal period has not been established for this product in pre-ruminating calves.
Do not use in dairy cows or in animals intended for subsequent breeding. Use in these cattle may cause drug residues in milk and/or in calves born to these cows.
Implant pellets subcutaneously in ear only. Any other location is a violation of Federal law. Do not attempt salvage of implanted site for human or animal food.
- USER SAFETY WARNINGS:
- ANIMAL SAFETY WARNINGS:
-
WARNINGS:
This product is intended for use in cattle at high risk of developing ear abscesses. Not recommended for use in cattle at low risk of developing ear abscesses (e.g., dry, clean cattle).
The benefit of using a cattle ear implant containing a tylosin tartrate pellet when animals receive a concomitant antimicrobial product has not been evaluated.
-
IMPLANTING INSTRUCTIONS:
Restrain the Animal
Speed of implantation as well as safety of handlers is best achieved by restraining animal in a squeeze chute using head restraint.
Prepare the Implant Site
Scrub the back side of the ear (implant site) with a piece of clean absorbent cotton or brush which has been soaked with topical germicidal solution. Follow manufacturer's directions on germicide for correct strength and preparation of solution. Avoid getting disinfectant in animal's eyes.
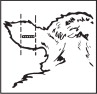
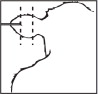
Where to Implant
The full contents of one cartridge cell should be implanted beneath the skin on the back side of the middle one-third of the ear as illustrated in the drawing. The implant must not be closer to the head than the edge of the auricular cartilage ring farthest from the head. The location for insertion of the needle is a point toward the tip of the ear and at least a needle length away from the intended deposition site. Avoid injuring the large arteries, veins and cartilage of the ear.
Insert the Needle
With one hand firmly grasp the ear. With the other hand insert needle point through the skin and ease forward on a lateral plane until the entire length of the needle is under the skin.
Implant the Pellets
After inserting the needle fully in the correct implant position, squeeze the trigger fully as the needle is withdrawn from the ear. This properly deposits the implant in the needle track. This procedure should prevent breakage or crushing of pellets if otherwise forced into contact with tough fibrous-tissue underlying the skin. The length and total contact area of a single dose are designed to permit absorption of the hormones after implantation to stimulate good weight gain. Broken or crushed pellets may lead to undesirable side effects such as bulling, rectal and vaginal prolapse, etc., as noted in the WARNINGS.
-
STORAGE CONDITIONS:
Store at controlled room temperature 15° to 30°C (59° to 86°F) DO NOT refrigerate – avoid excessive heat and humidity. Discard open foil pouches.
Approved by FDA under NADA # 110-315
Manufactured by a non-sterilizing process.
Component E-C with Tylan (progesterone and estradiol benzoate and tylosin tartrate implants) is covered by U.S. Patent No. 5,874,098
Component, Tylan, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2023 Elanco or its affiliates
Distributed by Elanco US Inc., Greenfield, IN 46140, USA
Tylosin: Product of United Kingdom
Revised: June 2023
PA104028X AH0507
- Principal Display Panel - Component E-C With Tylan Cartridge Label
-
Principal Display Panel - Component E-C With Tylan Pouch Label
Elanco™
Component™
™
E-C with Tylan™
(progesterone and estradiol benzoate and tylosin tartrate implant)
BEEF CALVES 45 DAYS OF AGE AND OLDER
AND WEIGHING UP TO 400 LBS
Each implant consists of 100 mg progesterone USP
and 10 mg estradiol benzoate and 29 mg tylosin tartrateCaution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
IMPLANTS FOR BEEF CALVES 45 DAYS OF AGE AND OLDER AND WEIGHING UP TO 400 LBS
- Principal Display Panel - Component E-C With Tylan Carton Label
-
INGREDIENTS AND APPEARANCE
COMPONENT E-C WITH TYLAN
progesterone, estradiol benzoate, tylosin tartrate implantProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:58198-0507 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength progesterone (UNII: 4G7DS2Q64Y) (progesterone - UNII:4G7DS2Q64Y) progesterone 100 mg estradiol benzoate (UNII: 1S4CJB5ZGN) (estradiol - UNII:4TI98Z838E) estradiol benzoate 10 mg tylosin tartrate (UNII: 5P4625C51T) (tylosin - UNII:YEF4JXN031) tylosin tartrate 29 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58198-0507-2 5 in 1 CARTON 1 1 in 1 POUCH 1 20 in 1 CARTRIDGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA110315 12/09/2013 Labeler - Elanco US Inc. (966985624)