Label: XUREA- urea cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 72678-034-01 - Packager: NATIONAL BIO GREEN SCIENCES LIMITED LIABILITY COMPANY
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 11, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
Xurea Description
Xurea Cream is a potent keratolytic emollient which is a gentle, yet potent, tissue softener for skin and/or nails.
Each gram of Xurea Cream contains:
ACTIVE: 39% Urea in a cream base of:
INACTIVES: Aqua (Deionized Water), Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Propylene Glycol, Dimethicone, Melaleuca Alternifolia (Tea Tree) Oil, Helianthus Annuus (Sunflower) Oil, Chamomilla Recutita (Chamomile) Extract, Carbomer, Triethanolamine, Phenoxyethanol, Ethylhexylglycerin.CHEMISTRY
Urea is a diamide of carbonic acid with the following chemical structure: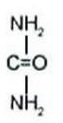
-
Xurea - Clinical Pharmacology
Urea gently dissolves the intercellular matrix which results in loosening the horny layer of skin and shedding scaly skin at regular intervals, thereby softening hyperkeratotic areas. Urea also hydrates and gently dissolves the intercellular matrix of the nail plate, which can result in the softening and eventual debridement of the nail plate.
- PHARMACOKINETICS
-
INDICATIONS AND USES
For debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or prurient debris or eschar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis, keratoderma, corns and calluses, as well as damaged, ingrown and devitalized nails.
- Contraindications
- Warnings
- Precautions
-
PREGNANCY
Pregnancy Category B
Animal reproduction studies have revealed no evidence of harm to the fetus, however, there are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, Xurea Cream should be given to a pregnant woman only if clearly needed.
- NURSING MOTHERS
- Adverse Reactions
-
Xurea - Dosage and Administration
Apply Xurea Cream to affected skin two to three times per day as needed or as directed by a physician. Rub in until completely absorbed. Apply to diseased or damaged nail tissue two to three times per day or as directed by a physician. Best applied to affected areas immediately after showering and just before bedtime.
-
How is Xurea Supplied
Xurea (39% Urea Cream) is supplied in:
8oz (227gm) Jar NDC 72678-034-01Store at 25°C (77°F); excursions permitted to 15°C - 30°C (59° - 86°F). Protect from freezing. [See USP Controlled Room Temperature.]
Manufactured for:
NATIONAL BIO GREEN SCIENCES LIMITED LIABILITY COMPANY
Branchburg, NJ 08876Rx only
-
SPL UNCLASSIFIED SECTION
PRINCIPAL DISPLAY PANEL - 227 gm Jar Label
Rx only
NDC 72678-034-01
Xurea UREA 39% CREAM
Smooth - Spreadable - Moisturizing
Xurea’s high amount of urea exhibits intensive keratolyic and peeling effects. Thoroughly nourishes, helping to prevent cracks, and drying of your skin.
FOR TOPICAL USE ONLY
Net WT. 8OZ (227 gm)
- Packaging
-
INGREDIENTS AND APPEARANCE
XUREA
urea creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72678-034 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UREA (UNII: 8W8T17847W) (UREA - UNII:8W8T17847W) UREA 390 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIMETHICONE (UNII: 92RU3N3Y1O) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) SUNFLOWER OIL (UNII: 3W1JG795YI) CHAMOMILE (UNII: FGL3685T2X) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) TROLAMINE (UNII: 9O3K93S3TK) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72678-034-01 227 g in 1 JAR; Type 0: Not a Combination Product 09/19/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/19/2019 Labeler - NATIONAL BIO GREEN SCIENCES LIMITED LIABILITY COMPANY (967054623)


