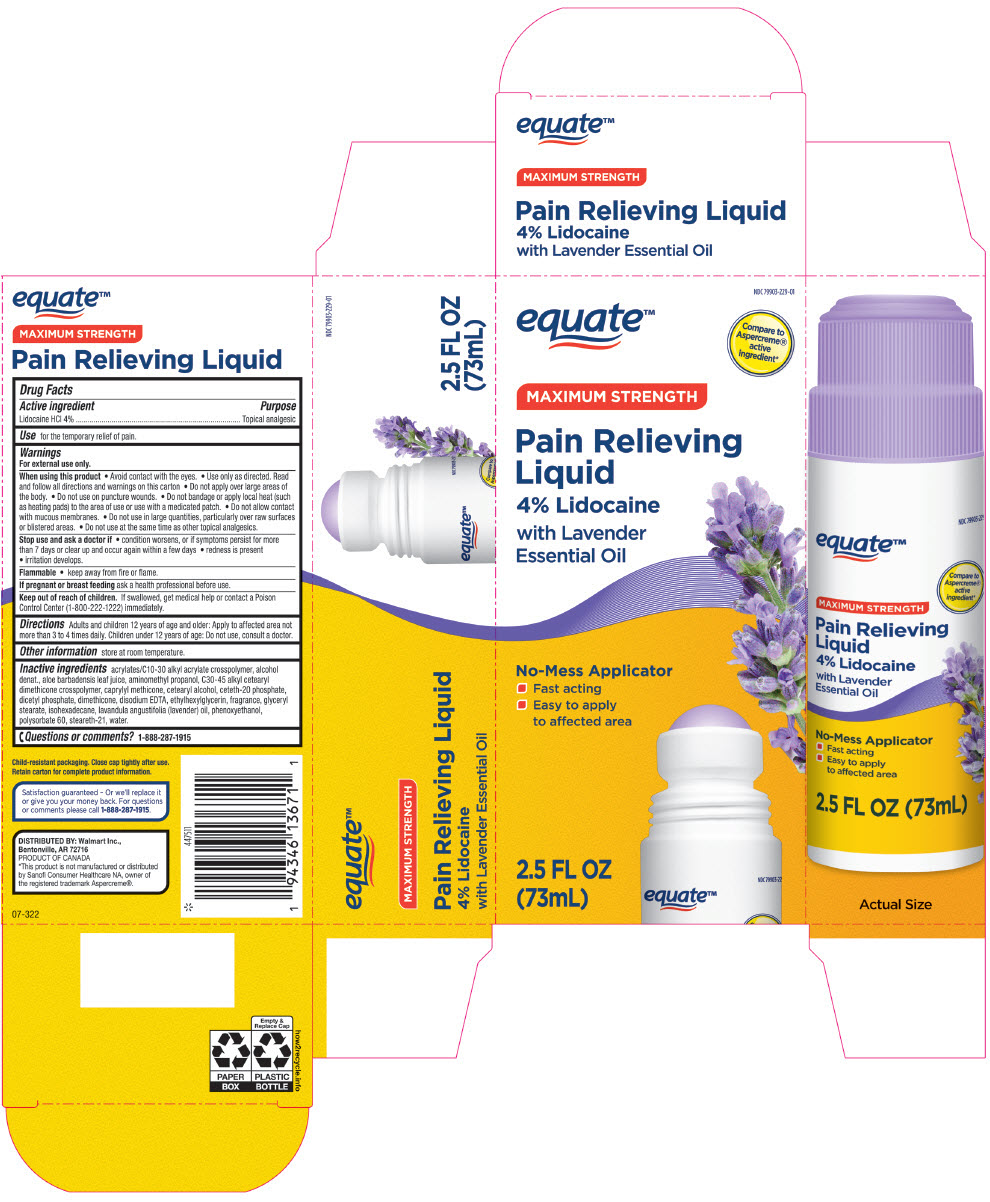
Label: EQUATE MAXIMUM STRENGTH PAIN RELIEVING 4% LIDOCAINE WITH LAVENDER ESSENTIAL OIL TOPICAL ANALGESIC- lidocaine hydrochloride liquid
- NDC Code(s): 79903-229-01
- Packager: Walmart Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 21, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only.
When using this product
- Avoid contact with the eyes.
- Use only as directed. Read and follow all directions and warnings on this carton
- Do not apply over large areas of the body.
- Do not use on puncture wounds.
- Do not bandage or apply local heat (such as heating pads) to the area of use or use with a medicated patch.
- Do not allow contact with mucous membranes.
- Do not use in large quantities, particularly over raw surfaces or blistered areas.
- Do not use at the same time as other topical analgesics.
- Directions
- Other information
-
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, alcohol denat., aloe barbadensis leaf juice, aminomethyl propanol, C30-45 alkyl cetearyl dimethicone crosspolymer, caprylyl methicone, cetearyl alcohol, ceteth-20 phosphate, dicetyl phosphate, dimethicone, disodium EDTA, ethylhexylglycerin, fragrance, glyceryl stearate, isohexadecane, lavandula angustifolia (lavender) oil, phenoxyethanol, polysorbate 60, steareth-21, water.
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 73 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
EQUATE MAXIMUM STRENGTH PAIN RELIEVING 4% LIDOCAINE WITH LAVENDER ESSENTIAL OIL TOPICAL ANALGESIC
lidocaine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-229 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) ALOE VERA LEAF (UNII: ZY81Z83H0X) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) C30-45 Alkyl Cetearyl Dimethicone Crosspolymer (UNII: 4ZK9VP326R) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) Ceteth-20 Phosphate (UNII: 921FTA1500) DIHEXADECYL PHOSPHATE (UNII: 2V6E5WN99N) Dimethicone (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) Ethylhexylglycerin (UNII: 147D247K3P) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) Isohexadecane (UNII: 918X1OUF1E) LAVENDER OIL (UNII: ZBP1YXW0H8) PHENOXYETHANOL (UNII: HIE492ZZ3T) Polysorbate 60 (UNII: CAL22UVI4M) Steareth-21 (UNII: 53J3F32P58) Water (UNII: 059QF0KO0R) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-229-01 1 in 1 CARTON 12/05/2023 1 73 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M017 12/05/2023 Labeler - Walmart Inc. (051957769) Registrant - Garcoa, Inc. (036464697) Establishment Name Address ID/FEI Business Operations Sigan Industries INC 255106239 MANUFACTURE(79903-229) , LABEL(79903-229) , PACK(79903-229)