Label: SECURA STARTER KIT- benzethonium chloride,petrolatum kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 69740-342-00 - Packager: Smith & Nephew Medical Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 14, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DIRECTIONS
- Change wet and soiled diapers, garments, and linens promptly. Spray cleanser liberally on affected area.
- Wipe clean with tissues, and remove soiled underpads and diapers.
- Immediately dispose of tissues and underpads to control odor. Allow to dry. No rinsing necessary.
- Apply protective ointment liberally as often as necessary with each diaper, garment, or linen change; especially at bedtime or anytime when exposure to soiled diapers, garments, linens, feces or urine may be prolonged.
- Check patient often. Repeat steps 1-4 after each exposure to feces and urine.
- Moisturize skin daily and after bathing to prevent dryness.
Refer to individual product labeling for detailed instructions.
Packaged in the USA with products made in the USA and India for:
Smith & Nephew Medical Ltd, 101 Hessle Road, Hull HU3 2BN England.
www.smith-nephew.com
Trademarks of Smith & Nephew
Certain marks reg’d U.S. Pat. and Tm. Off. - DRUG FACTS - SECURA MOISTURIZING CLEANSER ACTIVE INGREDIENT
- PURPOSE
- USES
- WARNING
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- INACTIVE INGREDIENTS
- DRUG FACTS - SECURA PROTECTIVE OINTMENT ACTIVE INGREDIENT
- PURPOSE
- USES
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
• change wet and soiled diapers, garments and linens promptly
• cleanse the affected area and allow to dry
• apply ointment liberally as often as necessary with each diaper, garment or linen change; especially at bedtime or anytime when exposure to soiled diapers, garments, linens, feces, or urine may be prolonged - INACTIVE INGREDIENTS
- QUESTIONS OR COMMENTS?
-
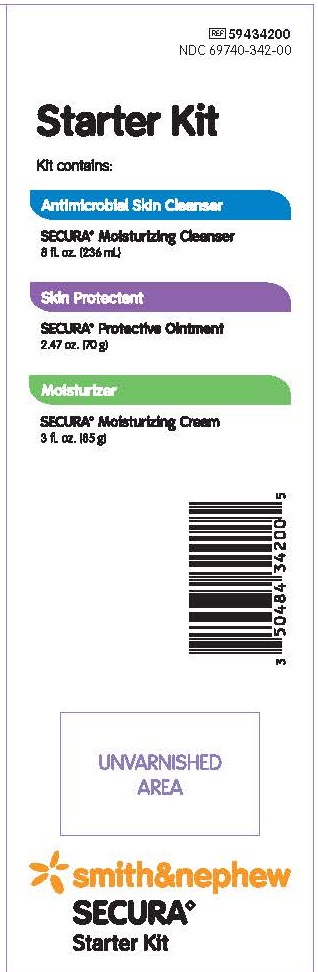
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - SECURA STARTER KIT
#59434200
NDC 69740-342-00Starter Kit
Kit Contains:
Antimicrobial Skin CleanserSecura Moisturizing Cleanser
8 fl oz (236 mL)
• No-rinse, one-step cleansing for perineum or body
• Gently removes urine, feces and other body fluids
• Reduces odor
• pH-buffered
• Pediatric tested
• CHG compatible
Skin ProtectantSecura Protective Ointment
2.47 oz (70 g)
• For treatment and prevention of incontinence-associated dermatitis
• Emriched with Vitamins A, D, & E
• Pediatric tested
• CHG compatible
• Enriched with Clove Oil
Moisturizer
Secura Moisturizing Cream
3 fl oz (85 g)
• Nourishes, hydrates and restores moisture to dry skin
• Enriched with Vitamin E
• Absorbs quickly, non-greasy
• Fortifies moisture balance
• Lanolin-free
• CHG compatible
Smith&Nephew
Secura
Starter Kit
- PACKAGE LABEL - SIDE DISPLAY PANEL - SECURA STARTER KIT
-
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - SECURA MOISTURIZING CLEANSER - BOTTLE (236 mL)
Item #59430900
NDC 69740-309-00
Moisturizing CleanserAntimicrobial Skin Cleanser
• No-rinse, one-step cleansing for perineum or body
• Gently removes urine, feces and other body fluids
• Reduces odor
• pH-balanced
• Pediatric tested
• CHG compatible
Smith & Nephew
Secura
Moisturizing Cleanser
8 fl. oz. (236 mL)
DRUG FACTS - SECURA? MOISTURIZING CLEANSER
ACTIVE INGREDIENTBenzethonium chloride 0.13%
PURPOSE
Antimicrobial
USES
• antimicrobial skin cleanser for the perineum or body
• aids in the removal of urine, feces, or other foreign materialWARNINGS
• For external use only
• Avoid contact with eyes
• Not to be applied over deep or puncture wounds, infections, or lacerations
• If condition worsens or does not improve within 7 days, consult a doctor
• Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately
DIRECTIONS• apply cleanser liberally on affected area, then wipe clean
• no rinsing necessaryINACTIVE INGREDIENTS
water, polysorbate 20, glycerin, polyquaternium-10, benzyl alcohol, fragrance, disodium EDTA, aloe barbadensis, FD&C Blue #1
QUESTIONS OR COMMENTS?1 800 876-1261
Made in USA for:
Smith & Nephew Medical Ltd,
101 Hessle Rd,
Hull HU3 2BN England.
www.smith-nephew.com
Trademark of Smith & Nephew.
Certain marks reg’d in U.S. Pat. and Tm. Off.
-
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - SECURA PROTECTIVE OINTMENT - TUBE (70 g)
Item #59431500
NDC 69740-315-00
Protective Ointment
Skin Protectant
• For treatment and prevention of incontinence-associated dermatitis
• Enriched with Vitamins A, D & E
• Pediatric tested
• CHG compatible
• Enriched with Clove Oil
Smith&Nephew
Secura
Protective OintmentNet wt. 2.47 oz. (70 g)
DRUG FACTS
ACTIVE INGREDIENT
Petrolatum 98.789%
PURPOSE
Skin protectant
USES
• skin protectant
• helps treat and prevent diaper dermatitis
• protects minor skin irritation associated with diaper dermatitis and helps seal out wetnessWARNINGS
• For external use only
• Avoid contact with the eyes
• Not to be applied over deep or puncture wounds, infections or lacerations
• If condition worsens or does not improve within 7 days, contact a doctor
• Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediatelyDIRECTIONS
• change wet and soiled diapers, garments and linens promptly
• cleanse the affected area and allow to dry
• apply ointment liberally as often as necessary with each diaper, garment or linen change; especially at bedtime or anytime when exposure to soiled diapers, garments, linens, feces, or urine may be prolongedINACTIVE INGREDIENTS
benzyl alcohol, clove oil, o-phenylphenol, tocopheryl acetate, corn oil, retinyl palmitate, cholecalciferol, D&C Green #6
QUESTIONS OR COMMENTS?
1 800 876-1261
Made in India for:
Smith & Nephew Medical Ltd.
101 Hessle Rd, Hull HU3 2BN England.Trademark of Smith & Nephew
Certain marks reg’d U.S. Pat. and Tm. Off.
-
INGREDIENTS AND APPEARANCE
SECURA STARTER KIT
benzethonium chloride,petrolatum kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69740-342 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69740-342-00 1 in 1 KIT 08/01/2003 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, SPRAY 236 mL Part 2 1 TUBE 70 g Part 1 of 2 SECURA MOISTURIZING CLEANSER
benzethonium chloride sprayProduct Information Item Code (Source) NDC:69740-309 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 1.3 ug in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA FLOWER (UNII: 575DY8C1ER) 0.05 ug in 1 mL BENZYL ALCOHOL (UNII: LKG8494WBH) 2 uL in 1 mL FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 0.001 uL in 1 mL GLYCERIN (UNII: PDC6A3C0OX) 10 uL in 1 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 30 ug in 1 mL WATER (UNII: 059QF0KO0R) 945.94 uL in 1 mL EDETATE DISODIUM (UNII: 7FLD91C86K) 0.2 ug in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 236 mL in 1 BOTTLE, SPRAY; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E Part 2 of 2 SECURA PROTECTIVE
petrolatum ointmentProduct Information Item Code (Source) NDC:69740-315 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 987.89 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) 5 mg in 1 g CLOVE OIL (UNII: 578389D6D0) 3 mg in 1 g D&C GREEN NO. 6 (UNII: 4QP5U84YF7) 0.01 mg in 1 g ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 2 mg in 1 g CHOLECALCIFEROL (UNII: 1C6V77QF41) 0.1 mg in 1 g VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) CORN OIL (UNII: 8470G57WFM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 70 g in 1 TUBE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 08/01/2003 Labeler - Smith & Nephew Medical Ltd (216344051) Establishment Name Address ID/FEI Business Operations Swiss-American Products, Inc. 080170933 manufacture(69740-309, 69740-342) Establishment Name Address ID/FEI Business Operations ENCUBE ETHICALS PVT LTD 915834105 manufacture(69740-315)