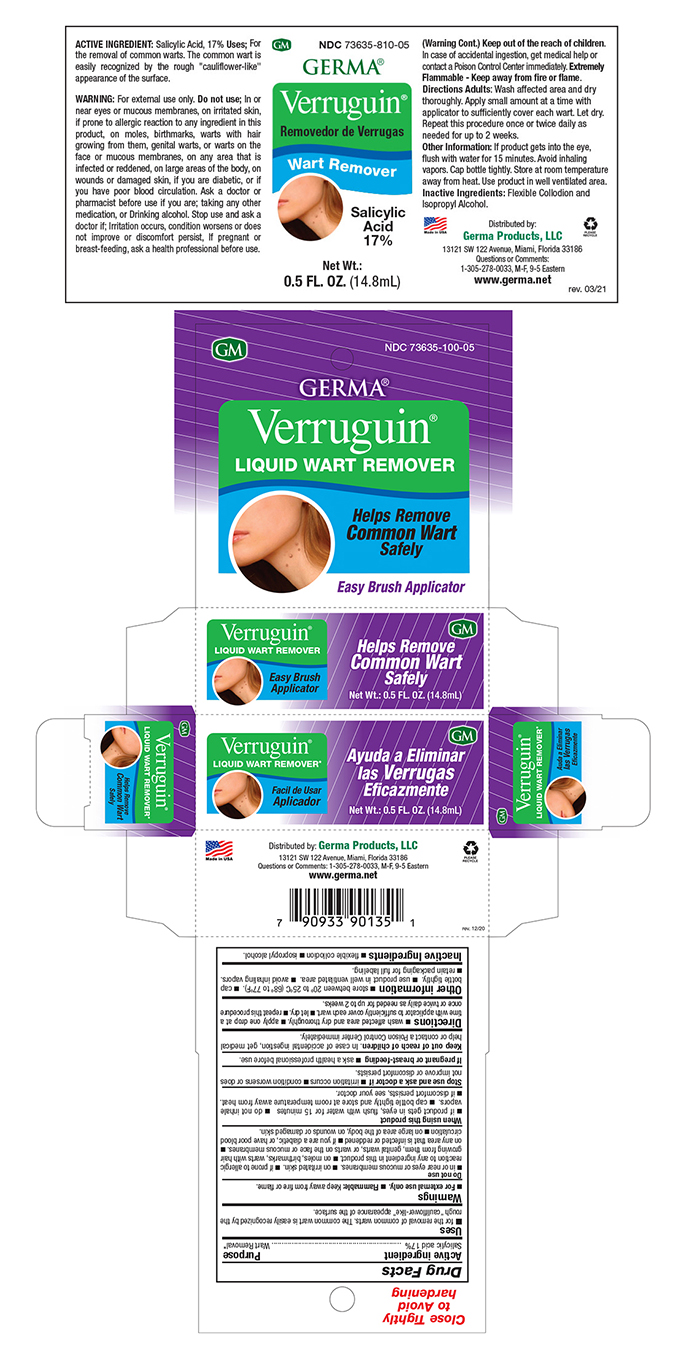
Label: VERRUGUIN LIQUID WART REMOVER- salisylic acid 17% liquid
- NDC Code(s): 73635-5810-5
- Packager: Germa Products, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Uses
- INDICATIONS & USAGE
- Purpose
-
Warnings
For external use only. Flammable: Keep away from fire or flame.
Do not use: in or near eyes or mucous membranes. On irritated skin. If prone to allergic reaction to any ingredient in this product. On moles, birthmarks, warts with hair growing from them, genital warts, or warts on the face or mucous membranes. On any area that is infected or reddened. If you are a diabetic, or have poor blood circulation n on large area of the body, on wounds or damaged skin.
When using this product: If product gets in eyes, flush with water for 15 minutes. Do not inhale vapors. Cap bottle tightly and store at room temperature away from heat.
If discomfort persists, see your doctor.
Stop use and ask a doctor if; Irritation occurs n condition worsens or does not improve or discomfort persists. If pregnant or breast-feeding. Ask a health professional before use. Keep out of reach of children. In case of accidental ingestion, get medical help or contact a Poison Control Center immediately. - Warnings
- Other Information
- Directions
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VERRUGUIN LIQUID WART REMOVER
salisylic acid 17% liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73635-5810 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 170 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) ALCOHOL (UNII: 3K9958V90M) CASTOR OIL (UNII: D5340Y2I9G) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73635-5810-5 1 in 1 BOX 03/29/2019 1 14.8 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M028 03/29/2019 Labeler - Germa Products, LLC (116626935)