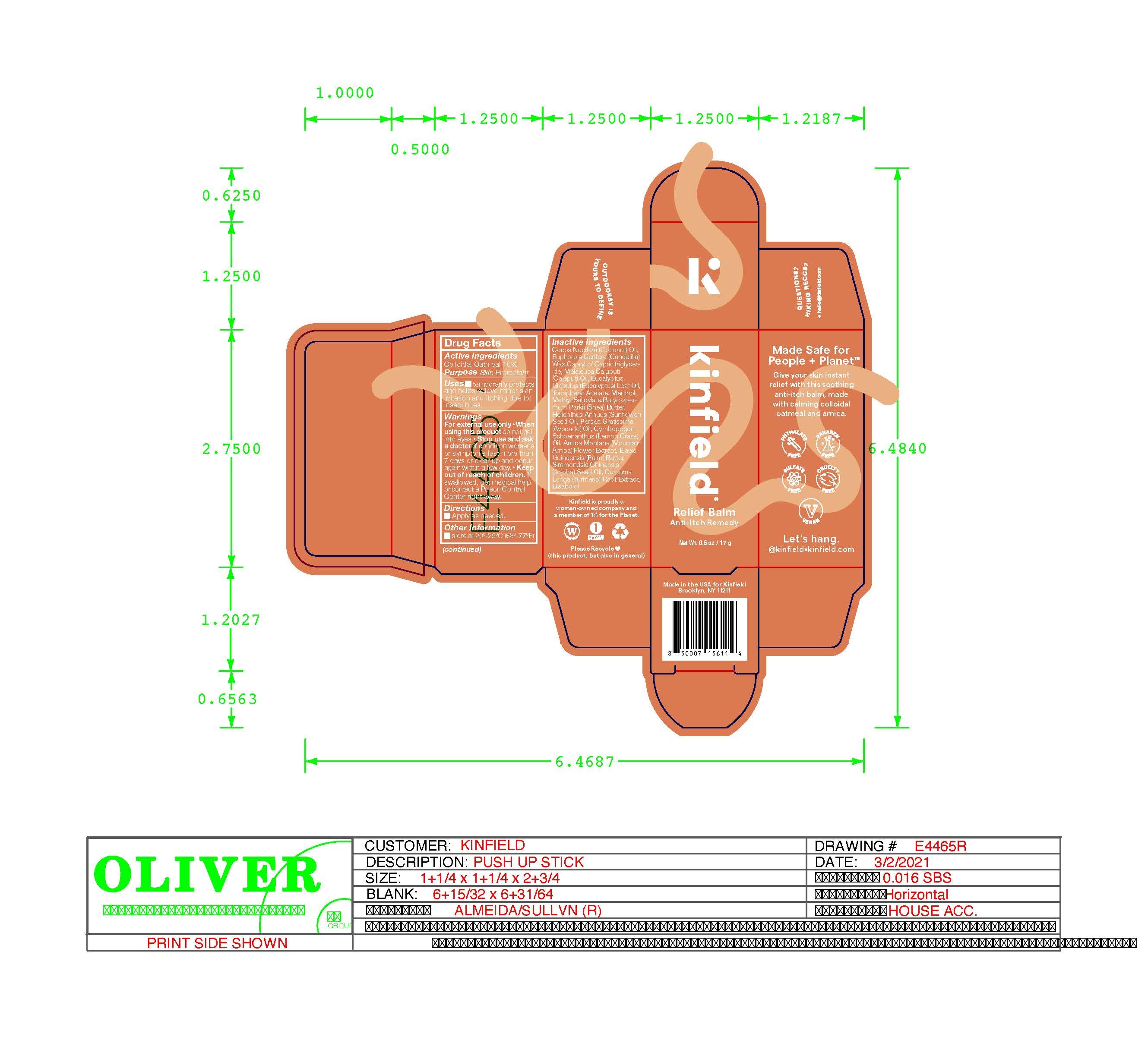
Label: KINFIELD RELIEF BALM ANTI-ITCH REMEDY lotion
- NDC Code(s): 81750-020-17, 81750-020-40
- Packager: Kinfield, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Purpose
- Active Ingredients
- Uses
- Warnings
- Keep out of reach of children. If swalled, get medical help or contact a Poison Control Center right away.
- Directions
-
Inactive Ingredients
Cocos Nucifera Oil, Euphorbia Cerifera Wax, Caprylic/Capric Triglyceride, Melaleuca Cajuputi Oil, Eucalyptus Globulus Leaf Oil, Tocopheryl Acetate, Menthol, Methyl Salicylate, Butyrospermum Parkii Butter, Helianthus Annuus Seed Oil, Persea Gratissima Oil, Cymbopogon Schoenanthus Oil, Arnica Montana Flower Extract, Elaeis Guineensis Butter, Simmondsia Chinensis Seed Oil, Curcuma Longa Root Extract, Bisabolol
- Other Information
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
KINFIELD RELIEF BALM ANTI-ITCH REMEDY
kinfield relief balm anti-itch remedy lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81750-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OATMEAL (UNII: 8PI54V663Y) (OATMEAL - UNII:8PI54V663Y) OATMEAL 10 g in 17 g Inactive Ingredients Ingredient Name Strength COCOS NUCIFERA WHOLE (UNII: 245J88W96L) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLIC/CAPRIC MONO/DI-GLYCERIDES (UNII: U72Q2I8C85) MELALEUCA CAJUPUTI WHOLE (UNII: K62C66D9LN) EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MENTHOL (UNII: L7T10EIP3A) METHYL SALICYLATE (UNII: LAV5U5022Y) SHEA BUTTER (UNII: K49155WL9Y) HELIANTHUS ANNUUS WHOLE (UNII: 17S27ZT6KR) PERSEA AMERICANA LEAF (UNII: 8490RQ2BET) CYMBOPOGON SCHOENANTHUS OIL (UNII: XE7K568ILO) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) ELAEIS GUINEENSIS WHOLE (UNII: UEG2DV5C8N) SIMMONDSIA CHINENSIS WHOLE (UNII: DFM16KFA82) CURCUMA LONGA WHOLE (UNII: W5488JUO8U) .BETA.-BISABOLOL (UNII: LP618AV2EA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81750-020-17 1 in 1 PACKAGE 04/05/2021 1 17 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:81750-020-40 1 in 1 PACKAGE 04/05/2021 2 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 04/05/2021 Labeler - Kinfield, Inc. (049511853)