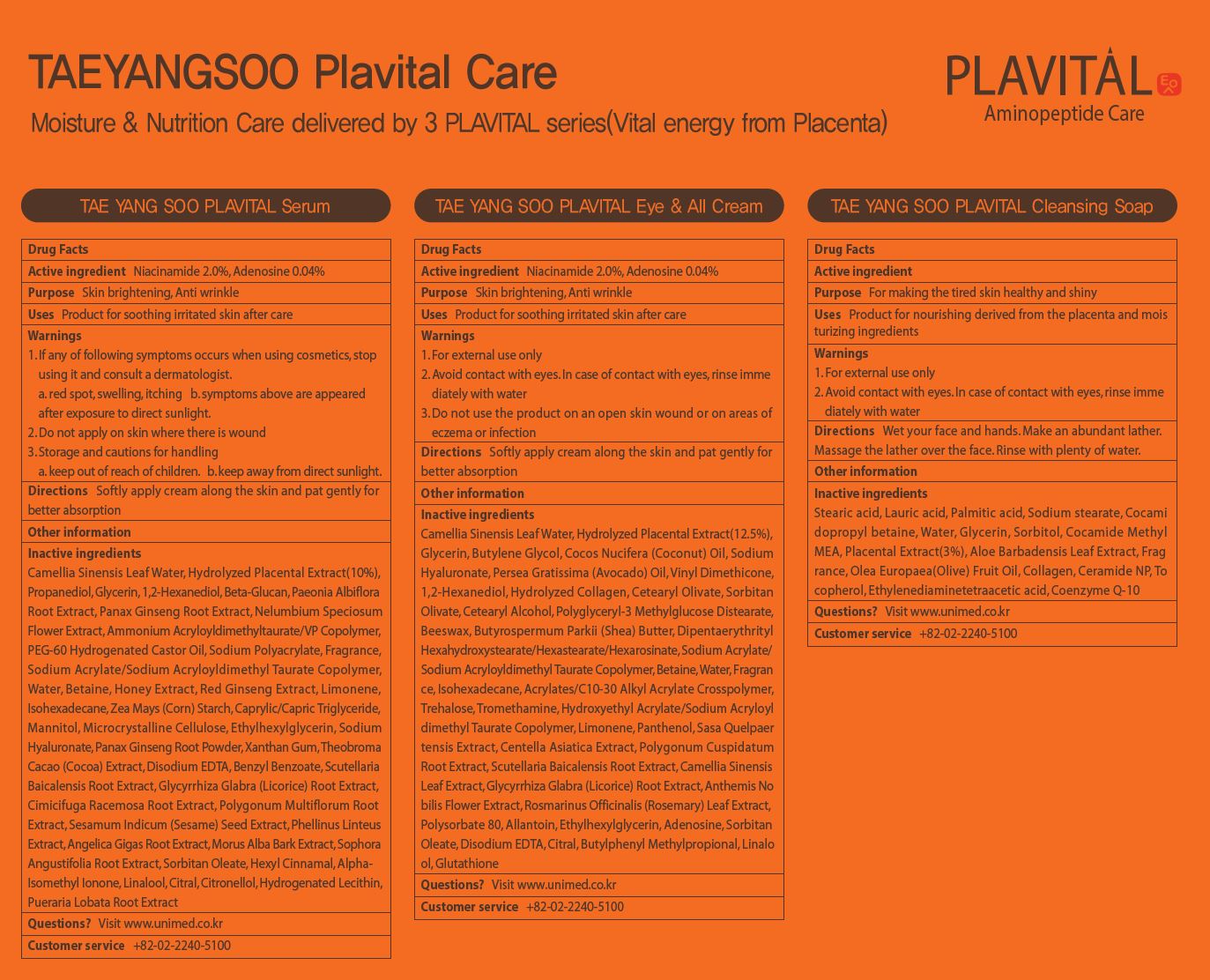
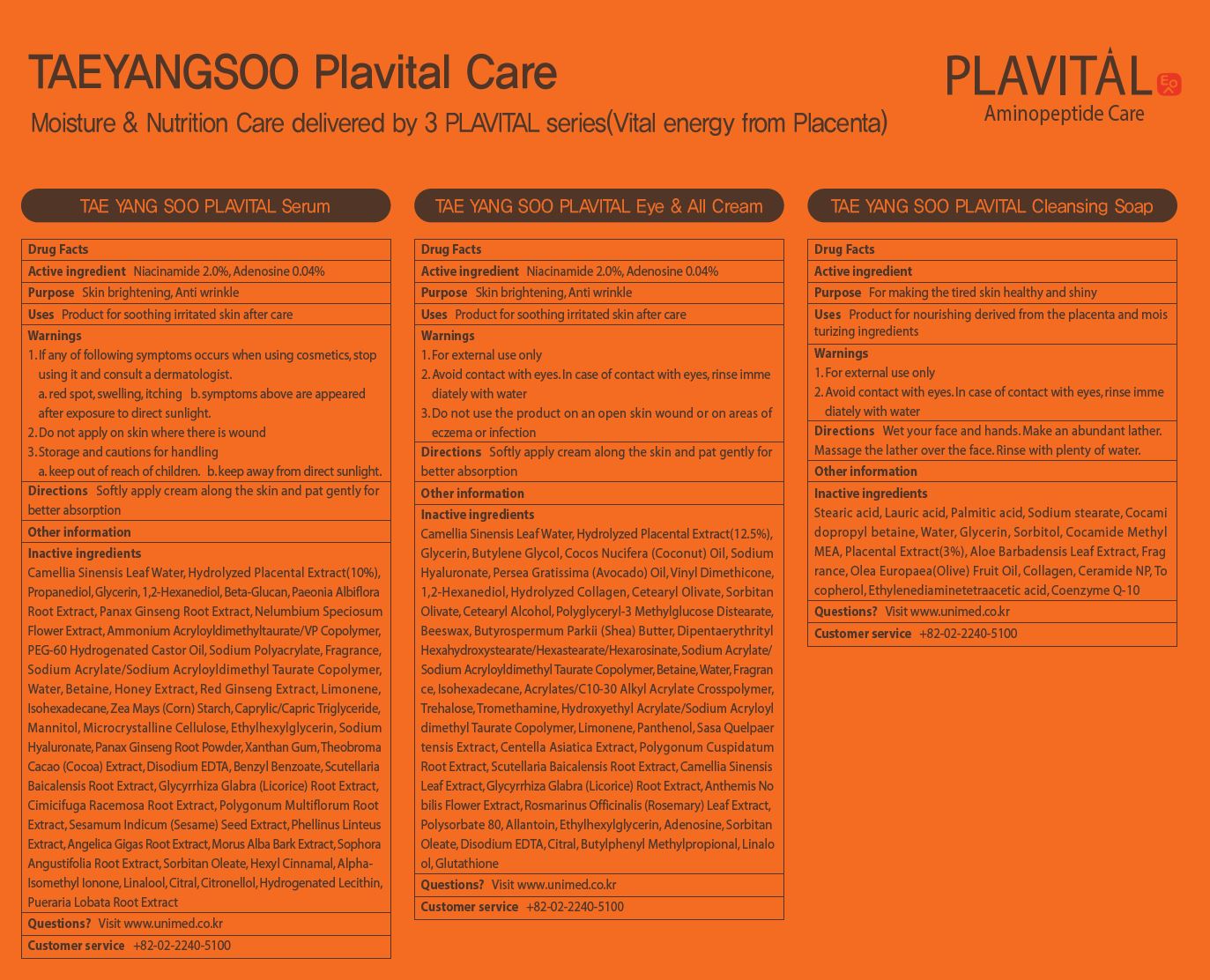
Label: TAEYANGSOO PLAVITAL EYE ALLCREAM- adenosine, niacinamide liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 73669-007-01 - Packager: Unimed Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 3, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Camellia Sinensis Leaf Water, Hydrolyzed Placental Extract(12.5%),
Glycerin, Butylene Glycol, Cocos Nucifera (Coconut) Oil, Sodium
Hyaluronate, Persea Gratissima (Avocado) Oil, Vinyl Dimethicone,
1,2-Hexanediol, Hydrolyzed Collagen, Cetearyl Olivate, Sorbitan
Olivate, Cetearyl Alcohol, Polyglyceryl-3 Methylglucose Distearate,
Beeswax, Butyrospermum Parkii (Shea) Butter, Dipentaerythrityl
Hexahydroxystearate/Hexastearate/Hexarosinate, Sodium Acrylate/
Sodium Acryloyldimethyl Taurate Copolymer, Betaine, Water, Fragran
ce, Isohexadecane, Acrylates/C10-30 Alkyl Acrylate Crosspolymer,
Trehalose, Tromethamine, Hydroxyethyl Acrylate/Sodium Acryloyl
dimethyl Taurate Copolymer, Limonene, Panthenol, Sasa Quelpaer
tensis Extract, Centella Asiatica Extract, Polygonum Cuspidatum
Root Extract, Scutellaria Baicalensis Root Extract, Camellia Sinensis
Leaf Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Anthemis No
bilis Flower Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract,
Polysorbate 80, Allantoin, Ethylhexylglycerin, Adenosine, Sorbitan
Oleate, Disodium EDTA, Citral, Butylphenyl Methylpropional, Linalo
ol, Glutathione - PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TAEYANGSOO PLAVITAL EYE ALLCREAM
adenosine, niacinamide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73669-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 2 g in 100 mL ADENOSINE (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) ADENOSINE 0.04 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73669-007-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/04/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/04/2021 Labeler - Unimed Pharmaceuticals, Inc. (689852052) Registrant - Unimed Pharmaceuticals, Inc. (689852052) Establishment Name Address ID/FEI Business Operations Unimed Pharmaceuticals, Inc. 689852052 label(73669-007) Establishment Name Address ID/FEI Business Operations C&t Dream Co., Ltd 694699750 manufacture(73669-007)