Label: ROC RETINOL CORREXION WRINKLE CORRECT DAILY MOISTURIZER SPF 30- avobenzone, homosalate, octisalate lotion
- NDC Code(s): 73496-004-01
- Packager: Roc Skincare
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
-
Directions for Sunscreen use
- Apply generously and evenly 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months of age: Ask a doctor
- Other information
-
INACTIVE INGREDIENT
Water, Glycerin, Diethylhexyl 2,6-Naphthalate, Dimethicone, Tetrahydroxypropyl Ethylenediamine, Butylene Glycol, Cyclohexasiloxane, Cetyl Alcohol, Butyrospermum Parkii (Shea) Butter, PPG-2 Myristyl Ether Propionate, Pentaerythrityl Tetraethylhexanoate, Squalane, Propanediol, Styrene/Acrylates Copolymer, Glyceryl Stearate, Aluminum Starch Octenylsuccinate, Retinol, Glycolic Acid, Tocopheryl Acetate, Ascorbyl Palmitate, Allantoin, p-Anisic Acid, Pentylene Glycol, Ceteth-20, Steareth-20, Polyacrylamide, PEG-8 Laurate, Laureth-7, C13-14 Isoparaffin, Ammonium Acryloyldimethyltaurate/VP Copolymer, PEG-75 Stearate, Cyclopentasiloxane, Polysorbate 20, Ascorbic Acid, Hydroxyphenyl Propamidobenzoic Acid, Caprylhydroxamic Acid, 1,2-Hexanediol, BHT, Disodium EDTA, Sodium Hydroxide, Fragrance.
- Questions or Comments?
-
INDICATIONS & USAGE
- Apply generously and evenly 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months of age: Ask a doctor
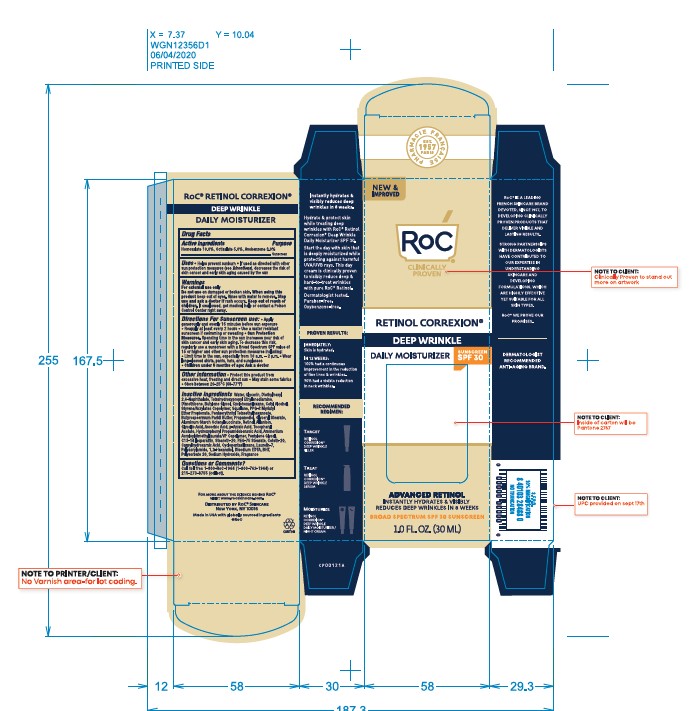
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ROC RETINOL CORREXION WRINKLE CORRECT DAILY MOISTURIZER SPF 30
avobenzone, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73496-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 mg in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 mg in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 mg in 100 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SODIUM HYDROXIDE (UNII: 55X04QC32I) EDETOL (UNII: Q4R969U9FR) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CETETH-20 (UNII: I835H2IHHX) LAURETH-7 (UNII: Z95S6G8201) WATER (UNII: 059QF0KO0R) DIETHYLHEXYL 2,6-NAPHTHALATE (UNII: I0DQJ7YGXM) SHEA BUTTER (UNII: K49155WL9Y) SQUALANE (UNII: GW89575KF9) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) GLYCOLIC ACID (UNII: 0WT12SX38S) ASCORBYL PALMITATE (UNII: QN83US2B0N) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) CETYL ALCOHOL (UNII: 936JST6JCN) ALLANTOIN (UNII: 344S277G0Z) PEG-8 LAURATE (UNII: 762O8IWA10) PEG-75 STEARATE (UNII: OT38R0N74H) PPG-2 MYRISTYL ETHER PROPIONATE (UNII: 88R97D8U8A) PENTAERYTHRITYL TETRAETHYLHEXANOATE (UNII: XJ7052W897) PROPANEDIOL (UNII: 5965N8W85T) RETINOL (UNII: G2SH0XKK91) P-ANISIC ACID (UNII: 4SB6Y7DMM3) PENTYLENE GLYCOL (UNII: 50C1307PZG) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) CYCLOMETHICONE 6 (UNII: XHK3U310BA) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) STEARETH-20 (UNII: L0Q8IK9E08) POLYSORBATE 20 (UNII: 7T1F30V5YH) ASCORBIC ACID (UNII: PQ6CK8PD0R) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROXYPHENYL PROPAMIDOBENZOIC ACID (UNII: 25KRT26H77) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73496-004-01 30 mL in 1 TUBE; Type 0: Not a Combination Product 04/05/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/02/2021 Labeler - Roc Skincare (117157981) Establishment Name Address ID/FEI Business Operations Dermaceutical Laboratories Limited Liability Company 078457159 manufacture(73496-004) Establishment Name Address ID/FEI Business Operations Amcol Health & Beauty Solutions, Incorporated 872684803 manufacture(73496-004)