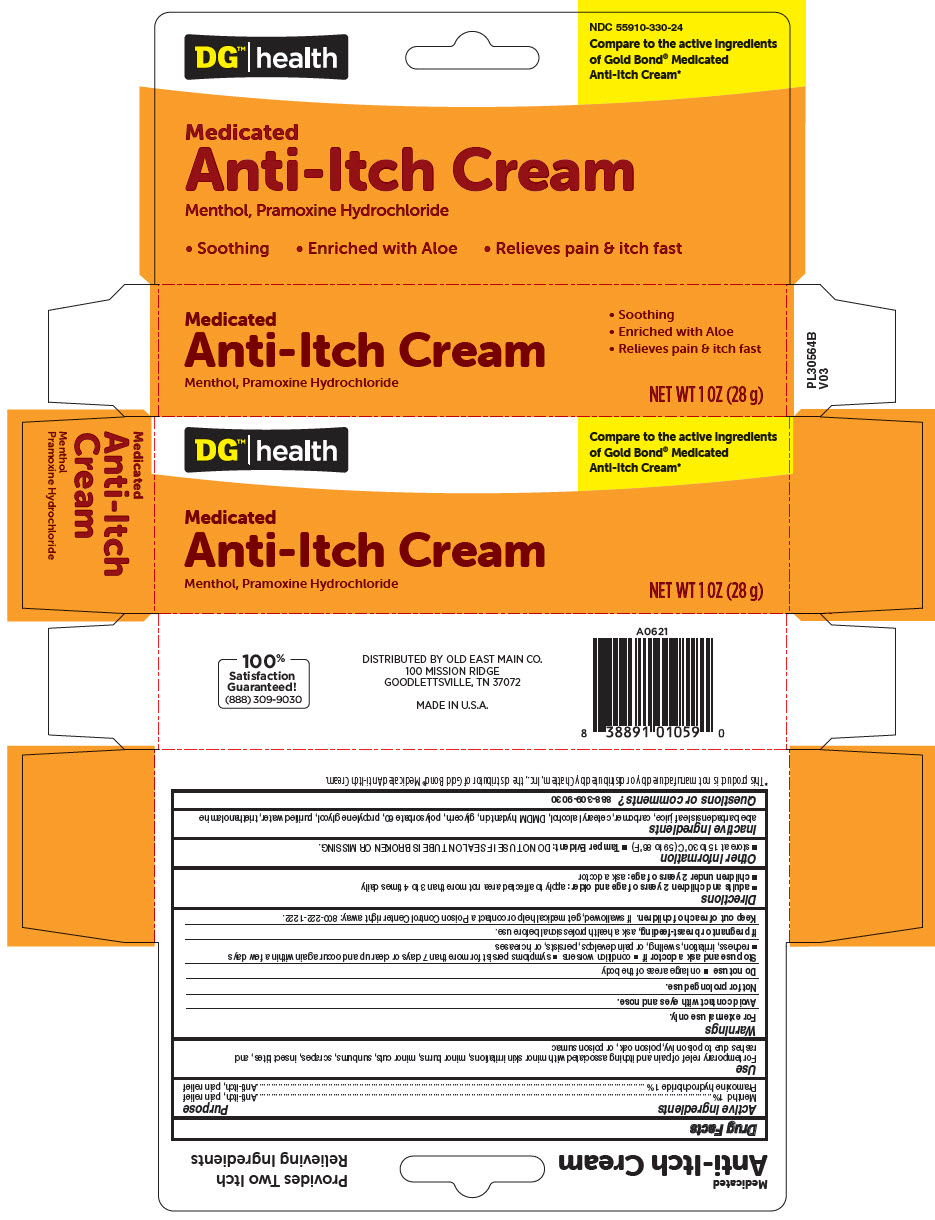
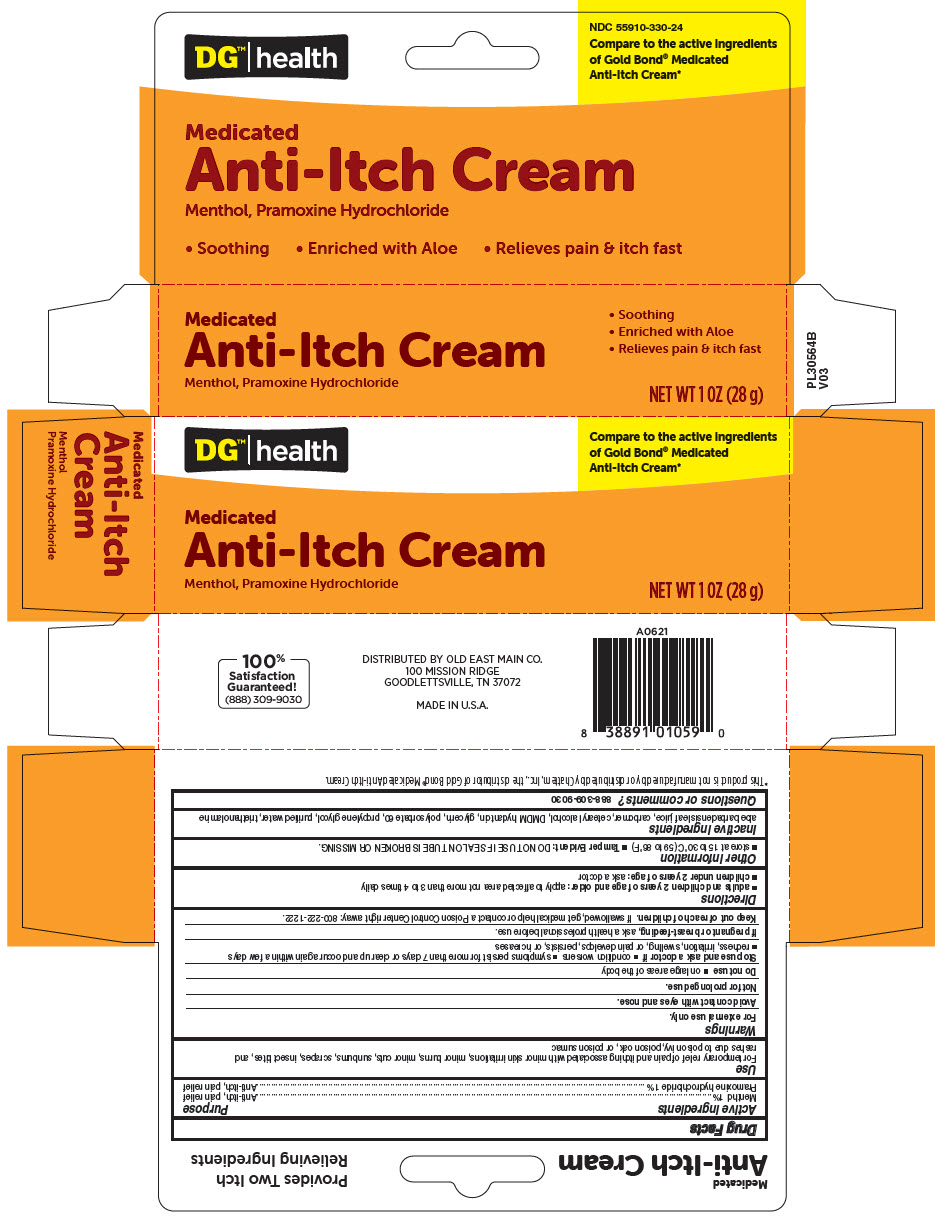
Label: DG HEALTH MEDICATED ANTI-ITCH ANALGESIC- menthol, unspecified form and pramoxine hydrochloride cream
- NDC Code(s): 55910-330-24
- Packager: DOLGENCORP, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 25, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Use
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 28 g Tube Carton
-
INGREDIENTS AND APPEARANCE
DG HEALTH MEDICATED ANTI-ITCH ANALGESIC
menthol, unspecified form and pramoxine hydrochloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55910-330 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Menthol, Unspecified Form (UNII: L7T10EIP3A) (Menthol, Unspecified Form - UNII:L7T10EIP3A) Menthol, Unspecified Form 10 mg in 1 g Pramoxine Hydrochloride (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) Pramoxine Hydrochloride 10 mg in 1 g Inactive Ingredients Ingredient Name Strength Aloe Vera Leaf (UNII: ZY81Z83H0X) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) DMDM HYDANTOIN (UNII: BYR0546TOW) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYSORBATE 60 (UNII: CAL22UVI4M) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55910-330-24 1 in 1 CARTON 01/02/2008 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part347 01/02/2008 Labeler - DOLGENCORP, LLC (068331990) Establishment Name Address ID/FEI Business Operations Natureplex LLC 062808196 MANUFACTURE(55910-330)