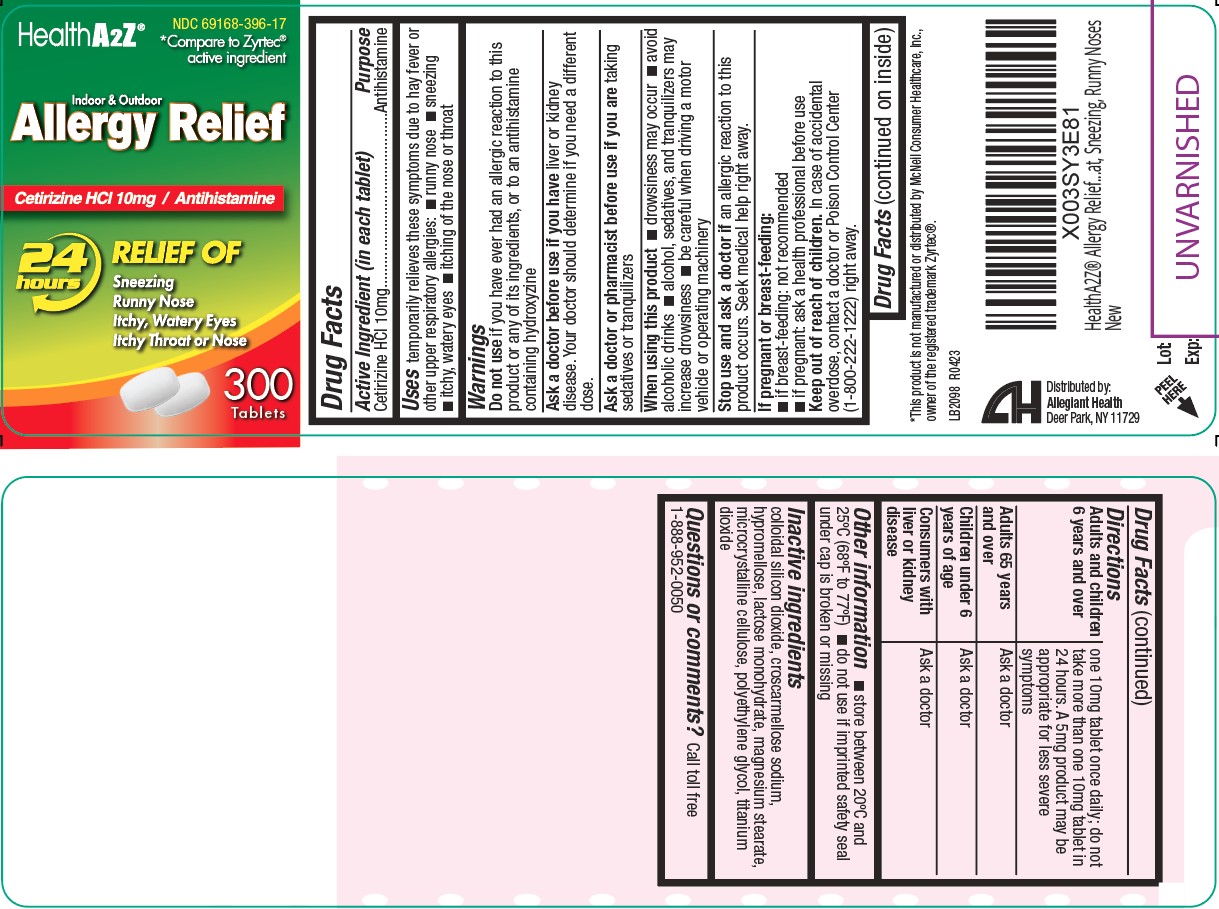
Label: ALLERGY RELIEF- cetirizine hcl 10 mg tablet
-
NDC Code(s):
69168-396-02,
69168-396-03,
69168-396-09,
69168-396-17, view more69168-396-30, 69168-396-43, 69168-396-60
- Packager: Allegiant Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 12, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine
Ask doctor if you have
- liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- Keep out of reach of children
-
Directions
- Adults and children 6 years and over: one 10mg tablet once daily; do not take more than one 10mg tablet in 24 hours. A 5mg product may be appropriate for less severe symptoms
- Adults 65 years and over: ask a doctor
- Children under 6 years of age: ask a doctor
- Consumers with liver or kidney disease: ask a doctor
- Other information
- Inactive Ingredients
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
cetirizine hcl 10 mg tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69168-396 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) Product Characteristics Color white Score 2 pieces Shape RECTANGLE (pillow-shaped) Size 9mm Flavor Imprint Code G;4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69168-396-09 1 in 1 CARTON 12/16/2014 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:69168-396-30 1 in 1 CARTON 12/16/2014 2 30 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:69168-396-03 250 in 1 BOTTLE; Type 0: Not a Combination Product 12/16/2014 4 NDC:69168-396-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 05/18/2022 5 NDC:69168-396-17 300 in 1 BOTTLE; Type 0: Not a Combination Product 05/31/2023 6 NDC:69168-396-43 45 in 1 BOTTLE; Type 0: Not a Combination Product 11/06/2023 7 NDC:69168-396-02 150 in 1 BOTTLE; Type 0: Not a Combination Product 11/06/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209274 12/16/2014 Labeler - Allegiant Health (079501930)