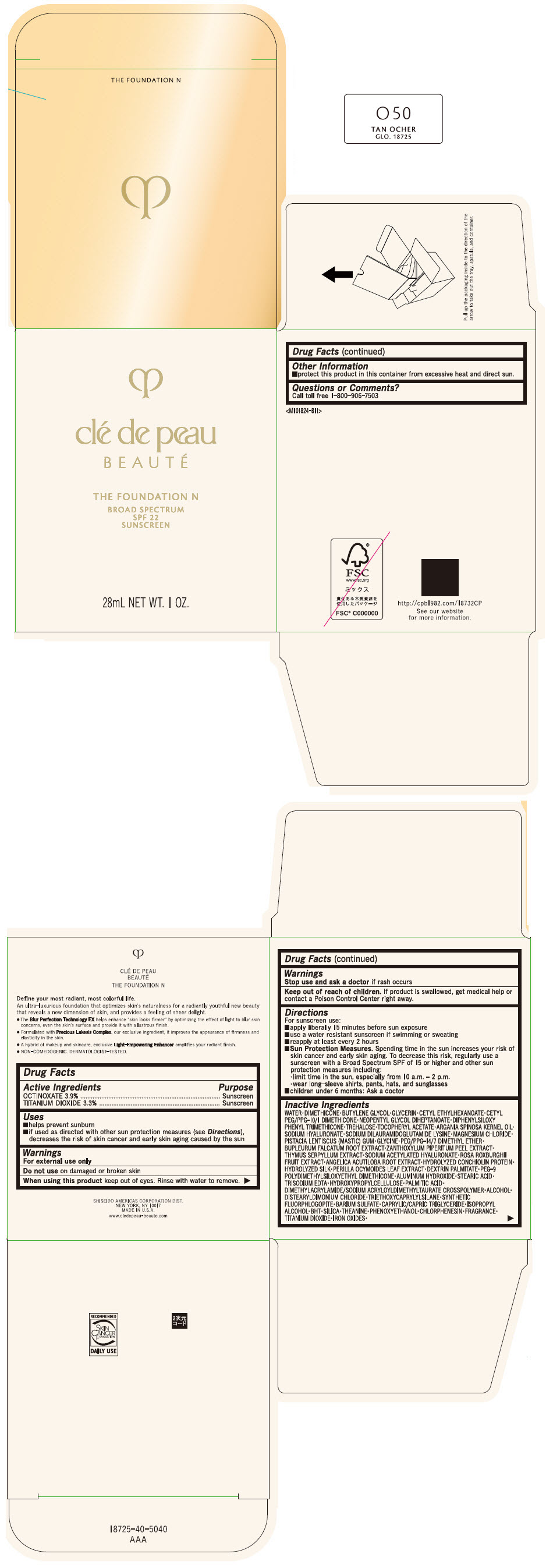
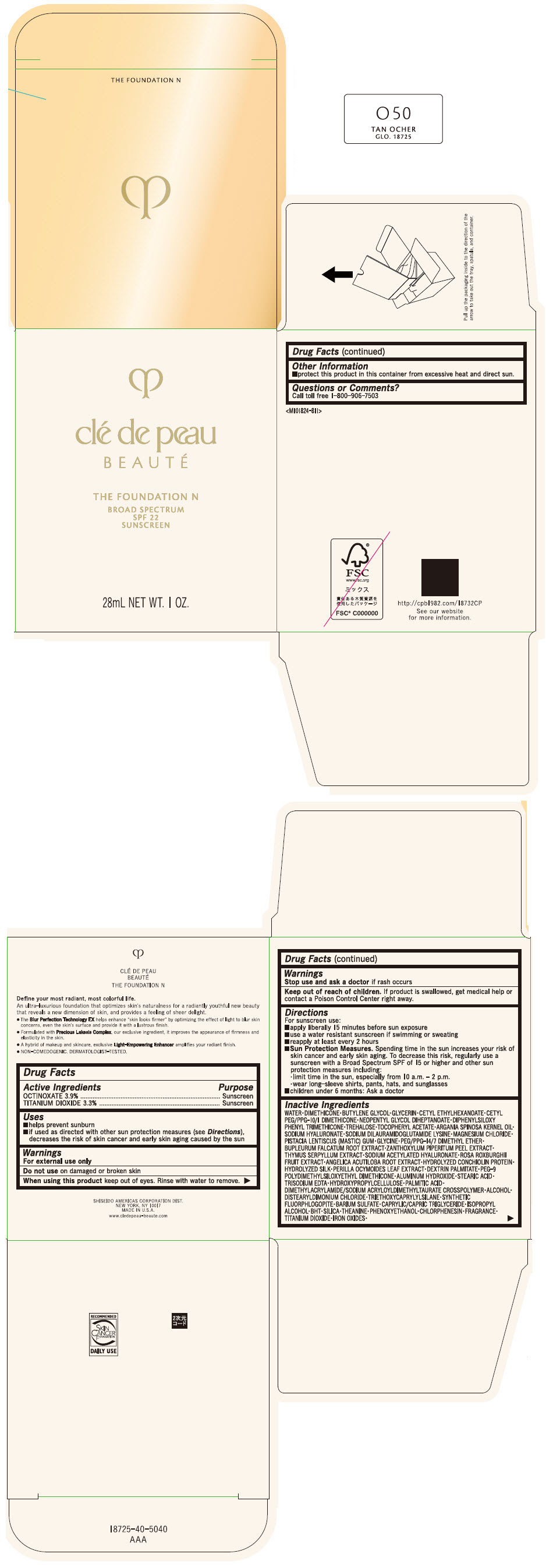
Label: CLE DE PEAU BEAUTE THE FOUNDATION N O50 TAN OCHER- octinoxate and titanium dioxide emulsion
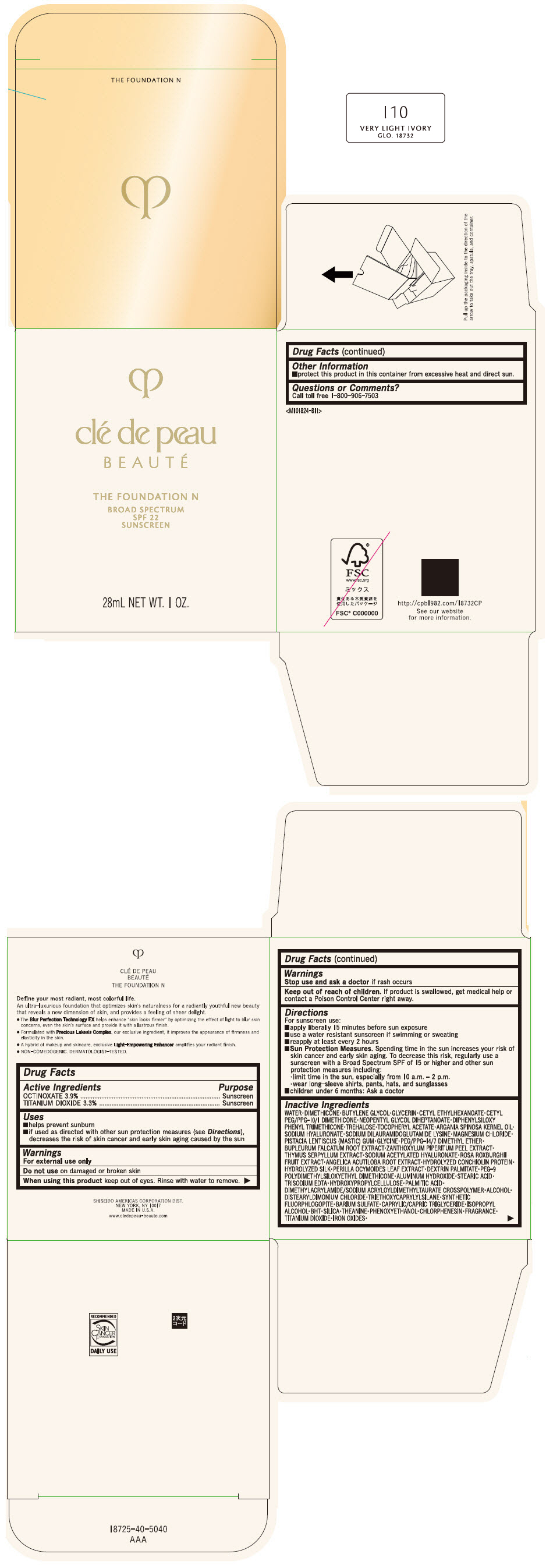
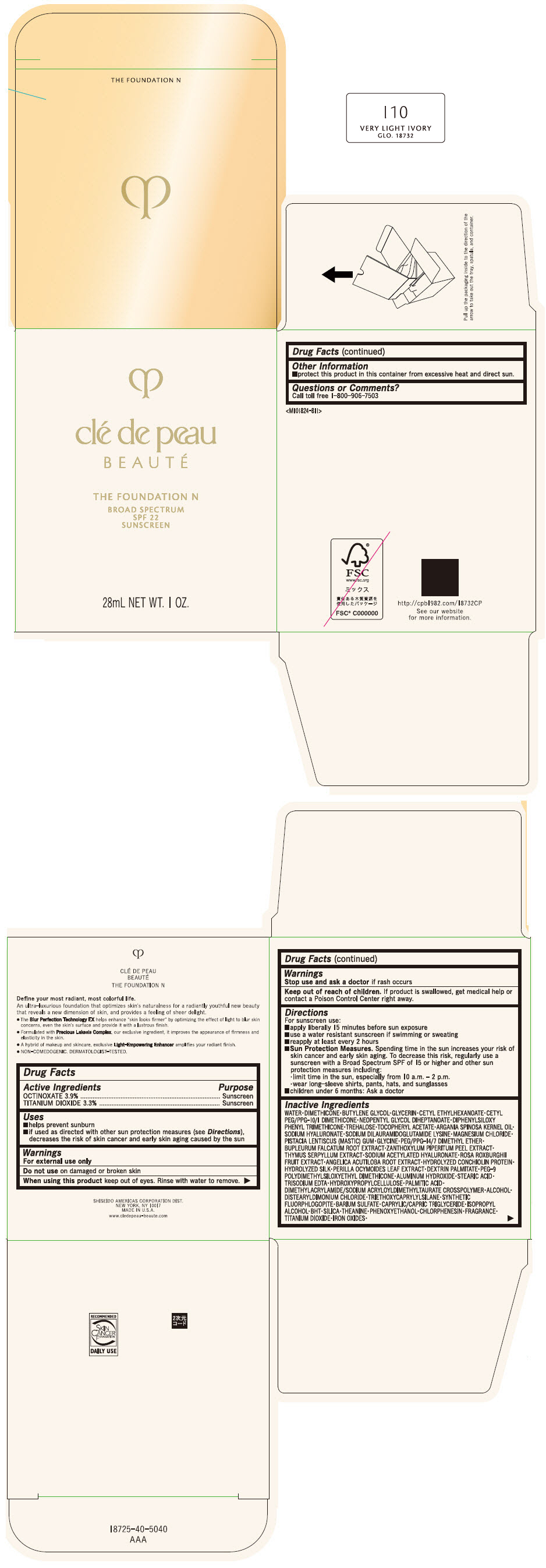
CLE DE PEAU BEAUTE THE FOUNDATION N I10 VERY LIGHT IVORY- octinoxate and titanium dioxide emulsion
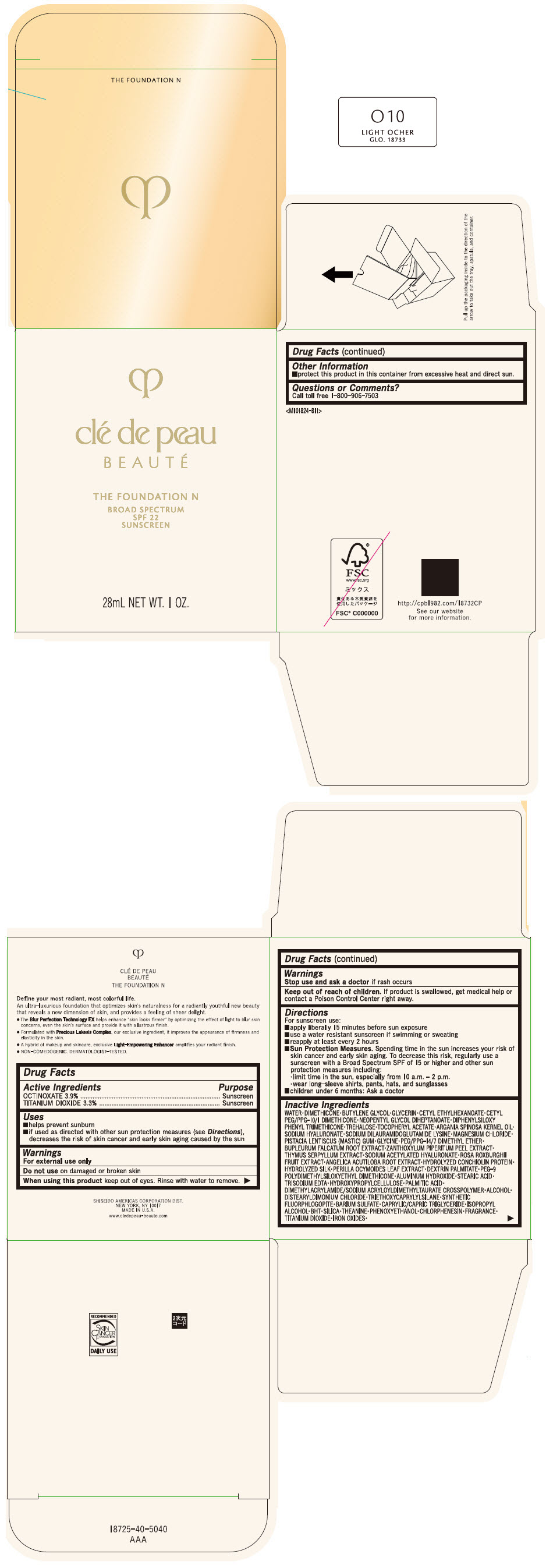
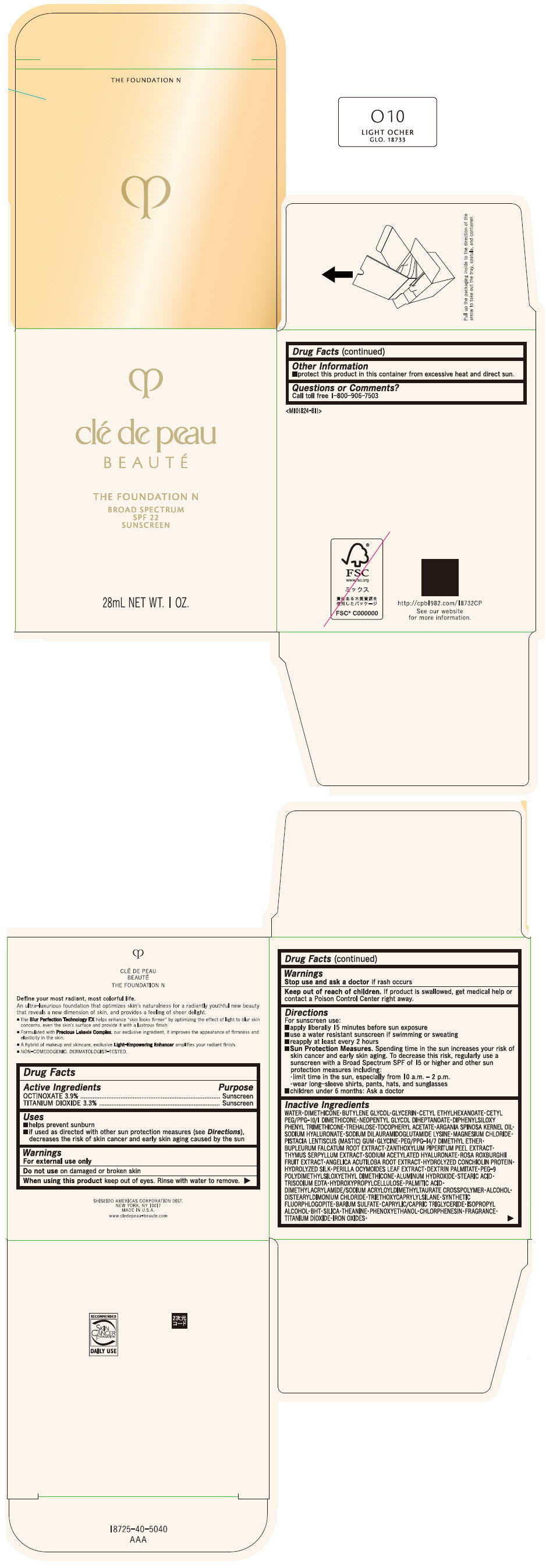
CLE DE PEAU BEAUTE THE FOUNDATION N O10 LIGHT OCHER- octinoxate and titanium dioxide emulsion
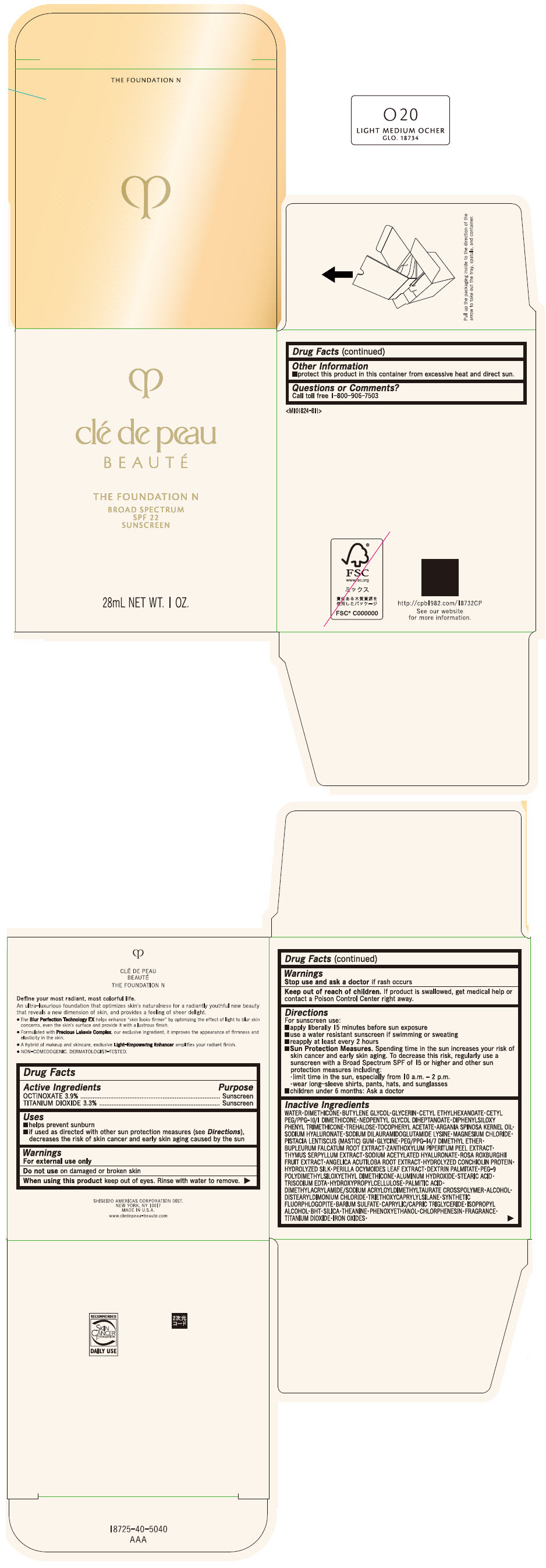
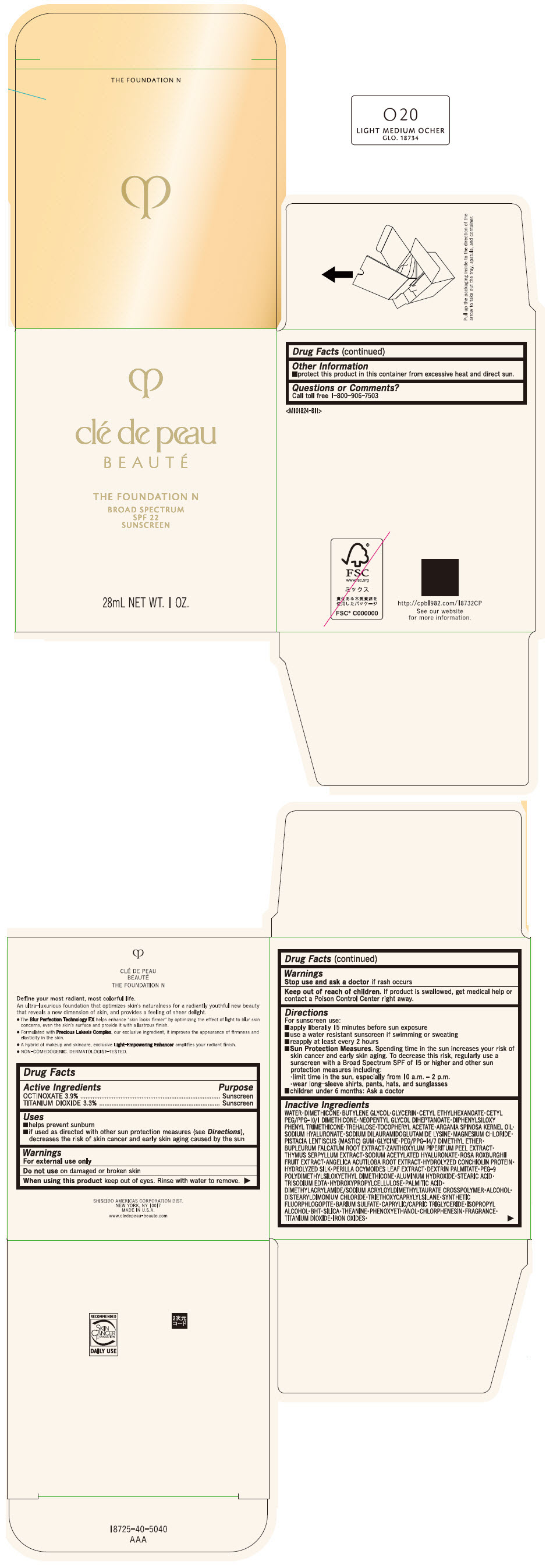
CLE DE PEAU BEAUTE THE FOUNDATION N O20 LIGHT MEDIUM OCHER- octinoxate and titanium dioxide emulsion
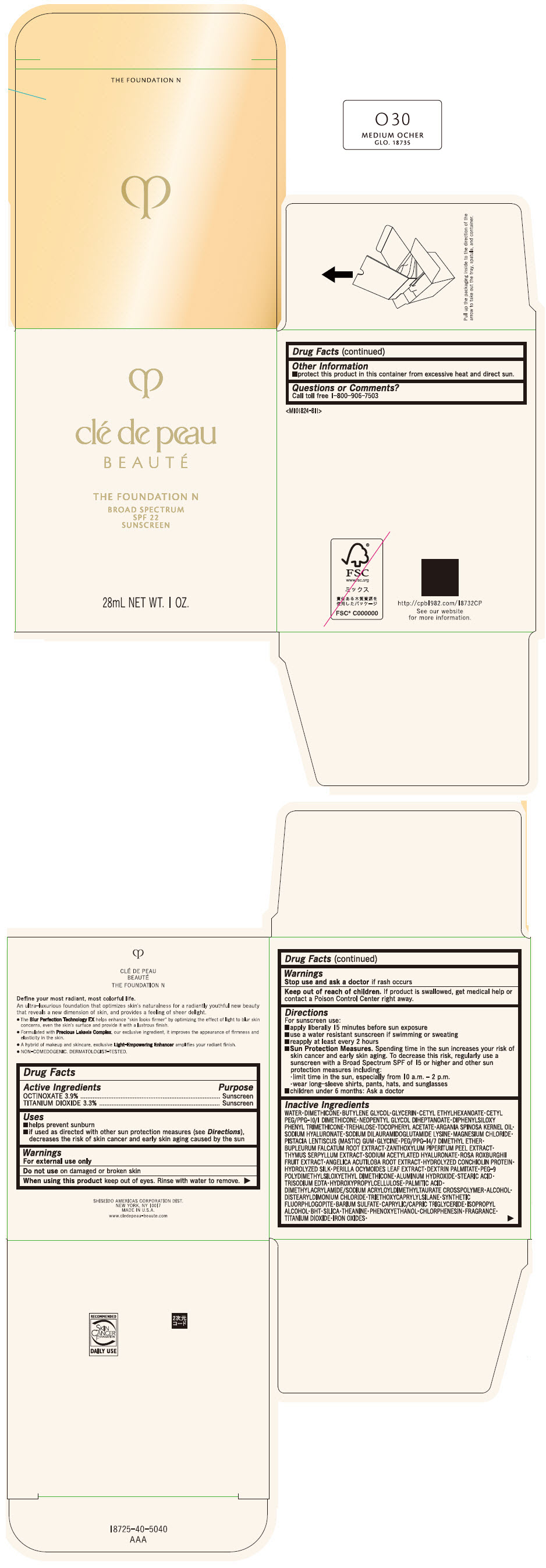
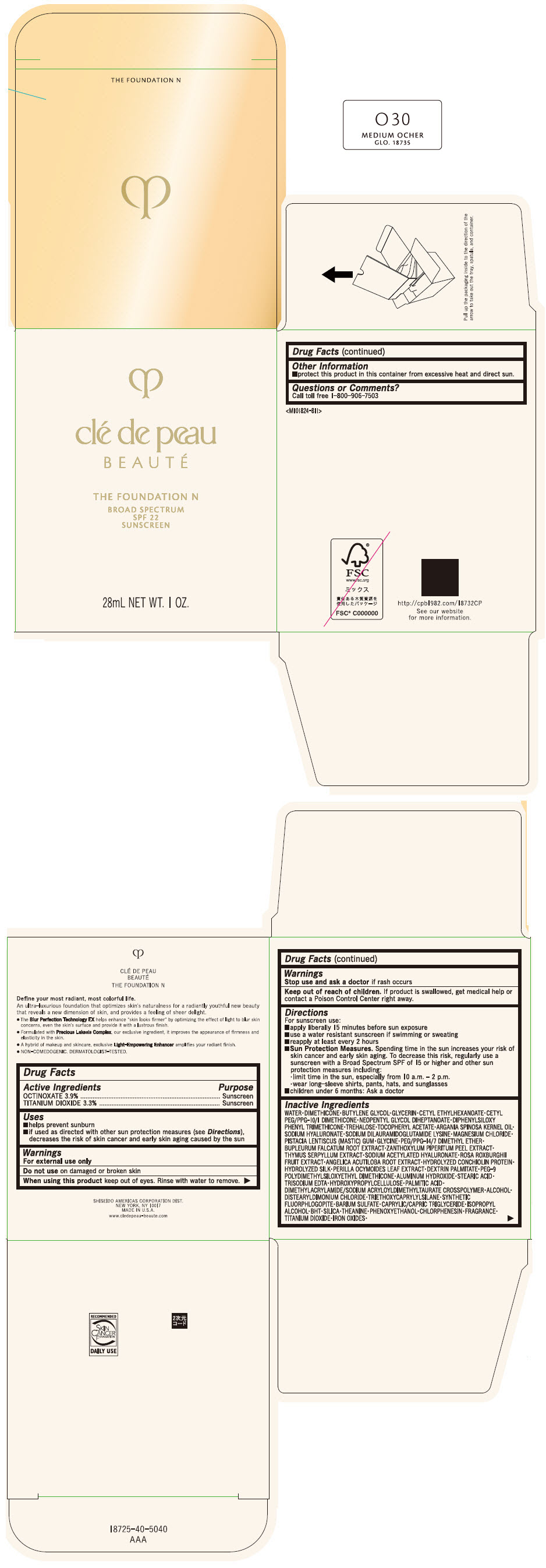
CLE DE PEAU BEAUTE THE FOUNDATION N O30 MEDIUM OCHER- octinoxate and titanium dioxide emulsion
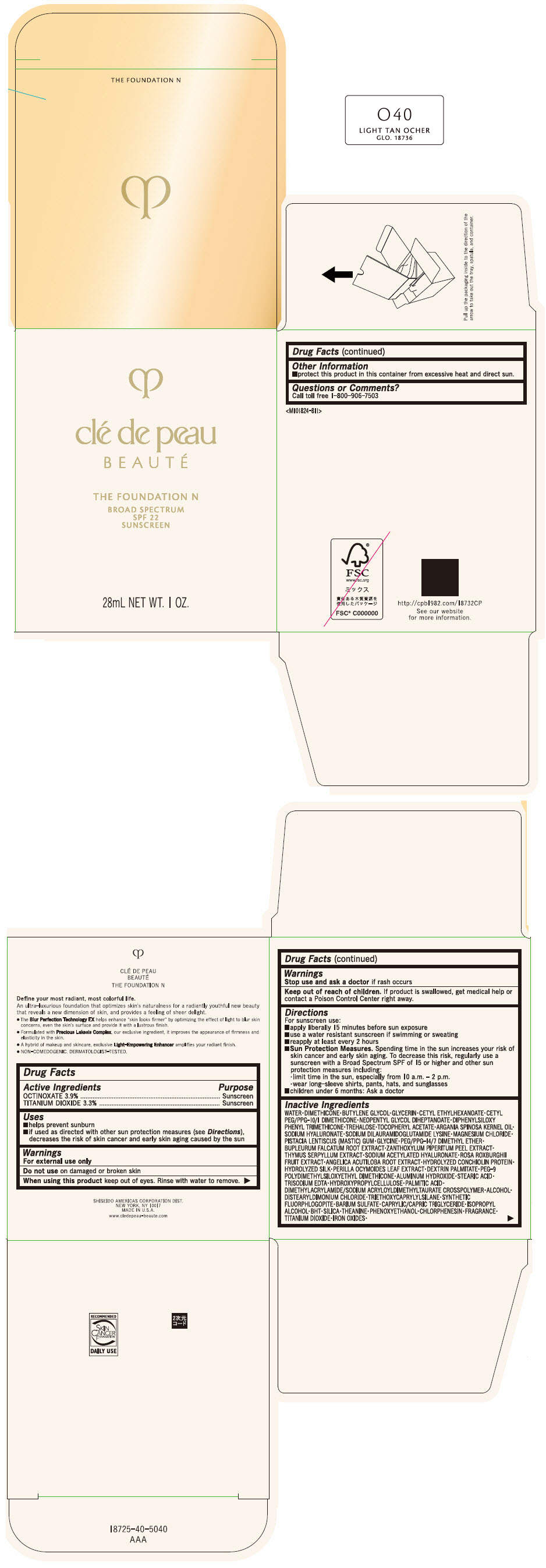
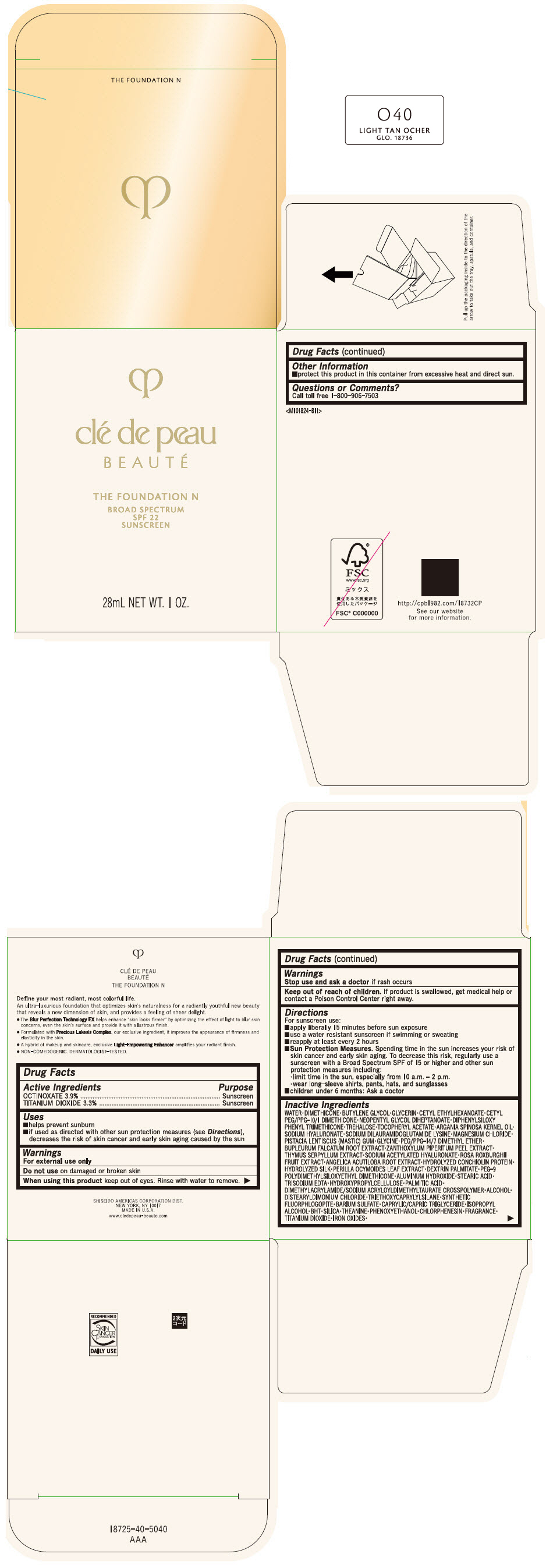
CLE DE PEAU BEAUTE THE FOUNDATION N O40 LIGHT TAN OCHER- octinoxate and titanium dioxide emulsion
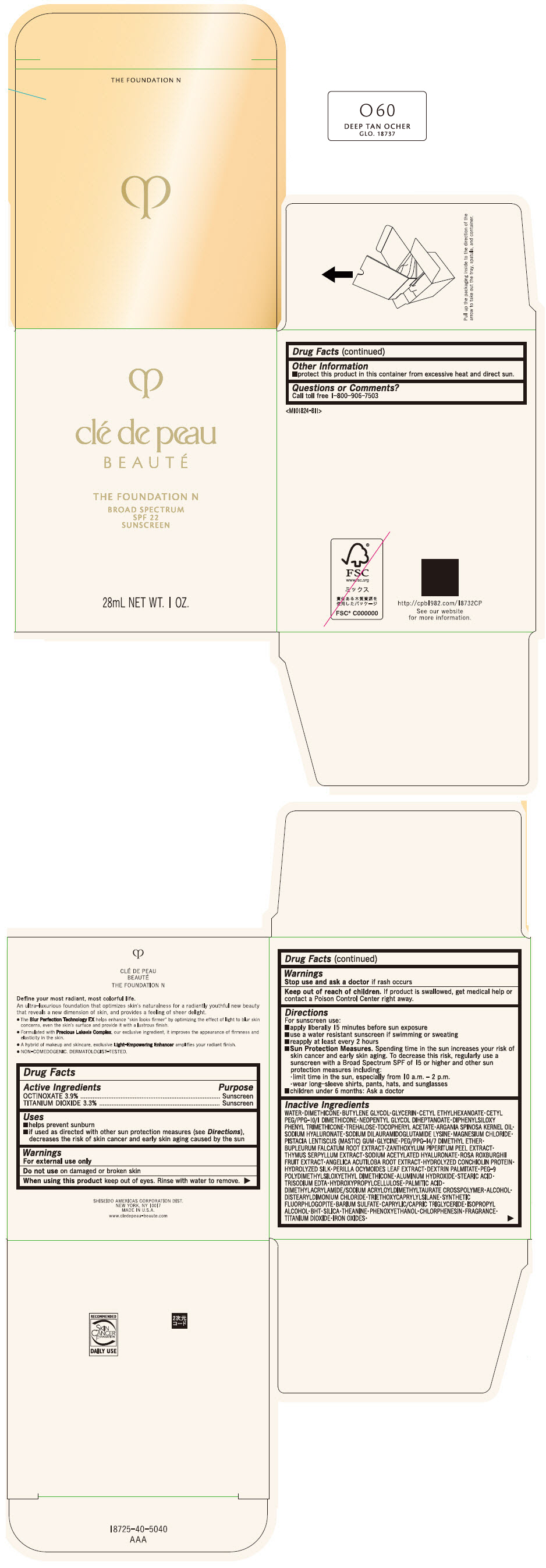
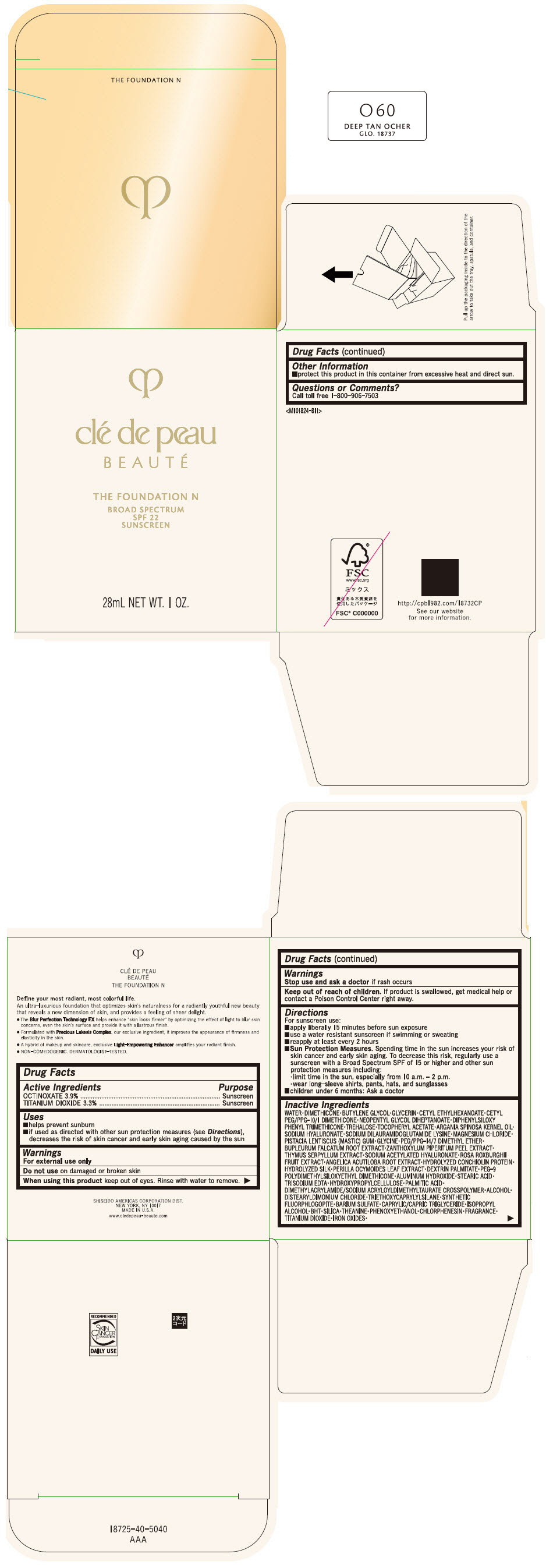
CLE DE PEAU BEAUTE THE FOUNDATION N O60 DEEP TAN OCHER- octinoxate and titanium dioxide emulsion
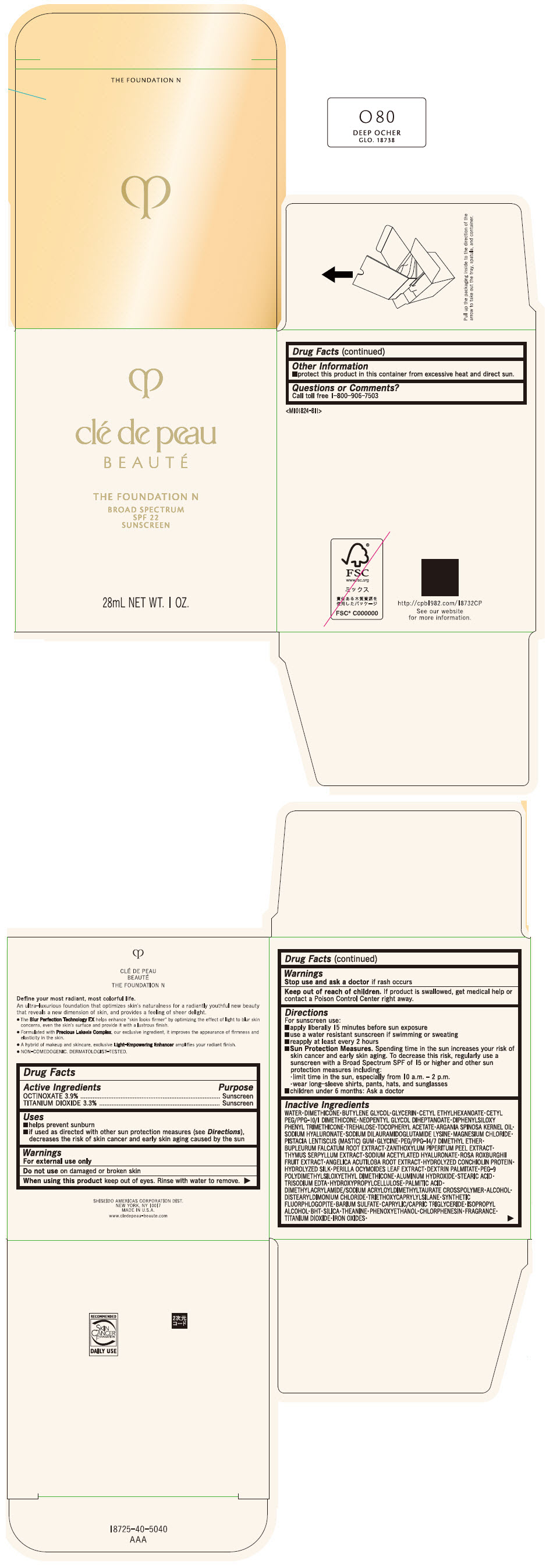
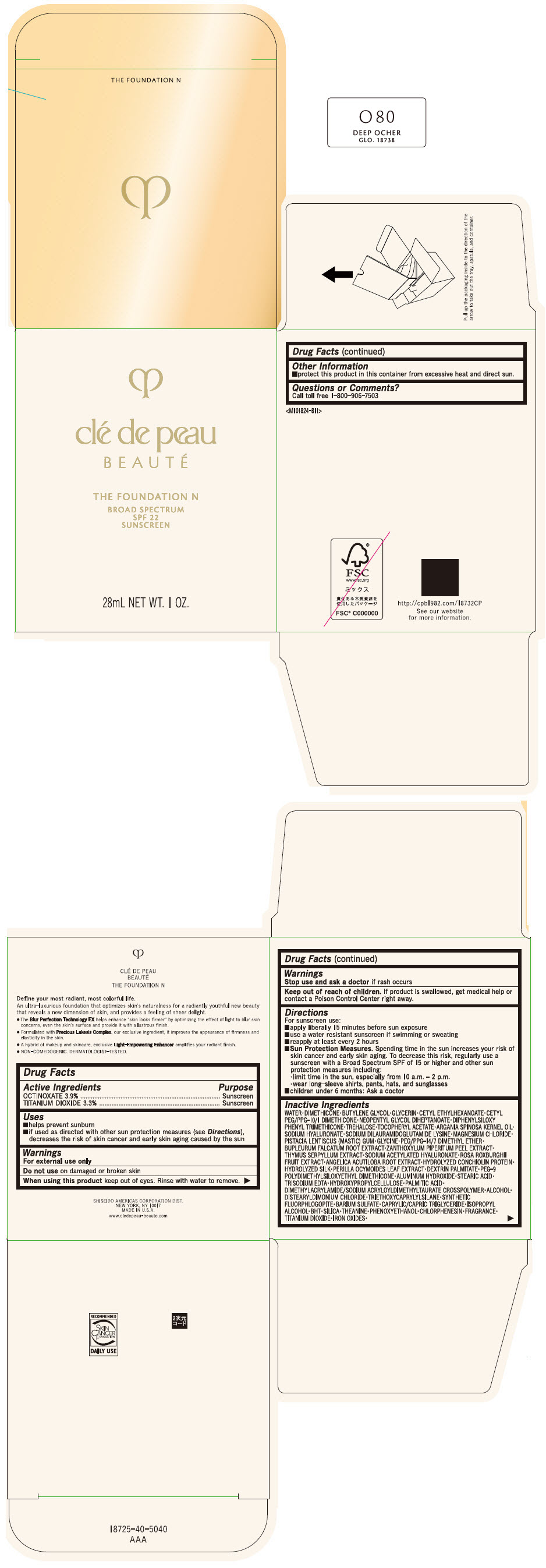
CLE DE PEAU BEAUTE THE FOUNDATION N O80 DEEP OCHER- octinoxate and titanium dioxide emulsion
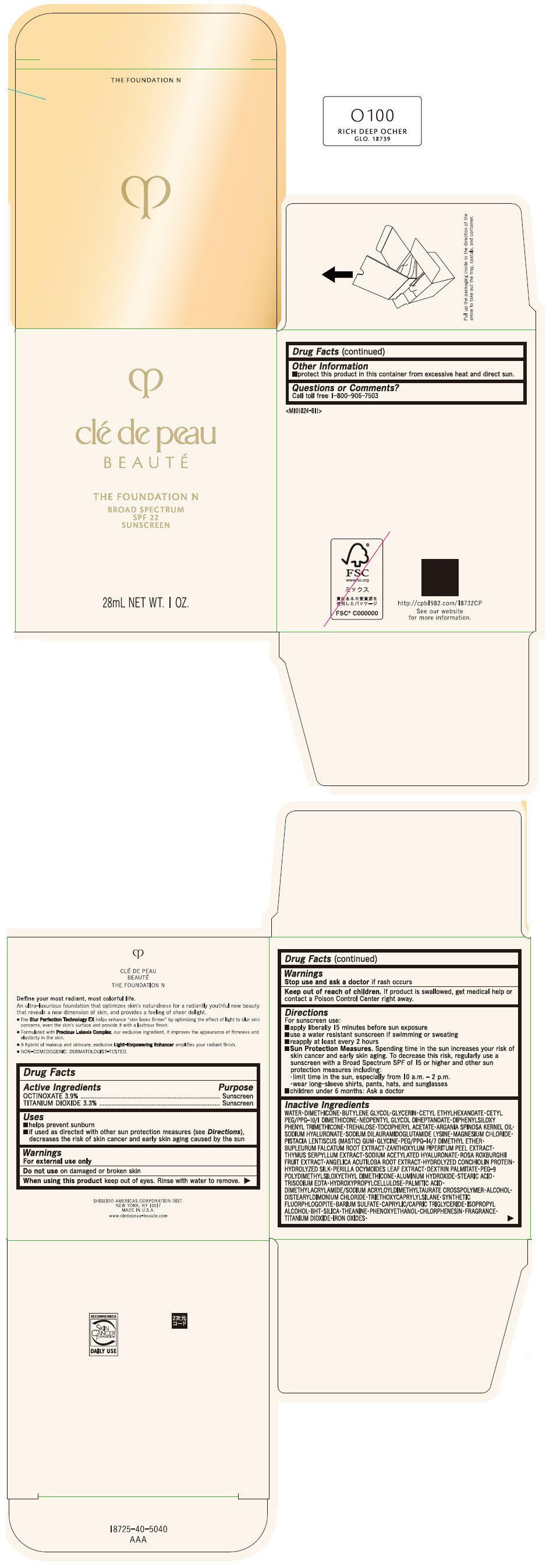
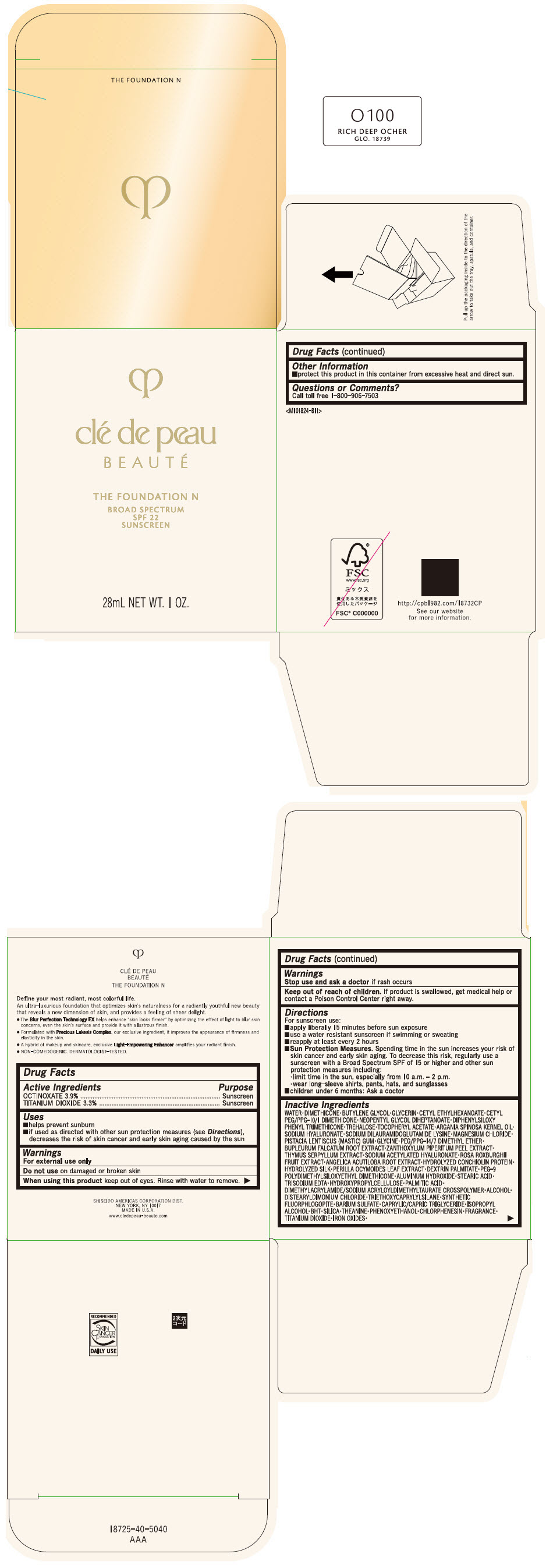
CLE DE PEAU BEAUTE THE FOUNDATION N O100 RICH DEEP OCHER- octinoxate and titanium dioxide emulsion
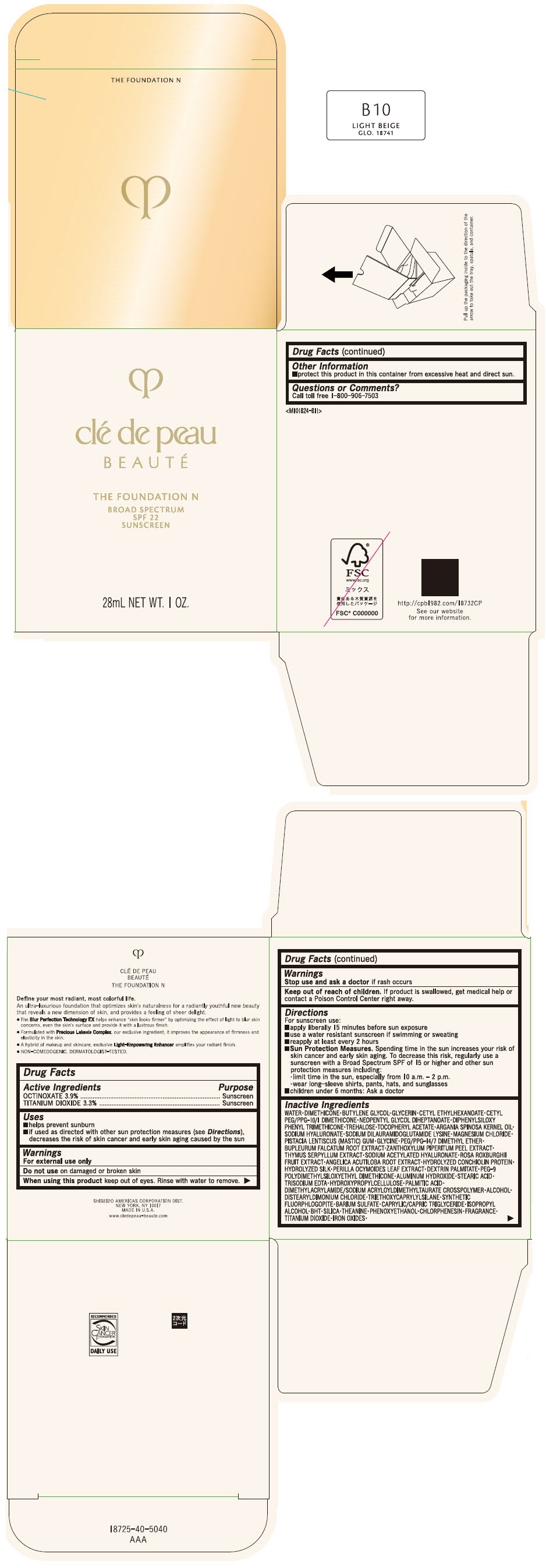
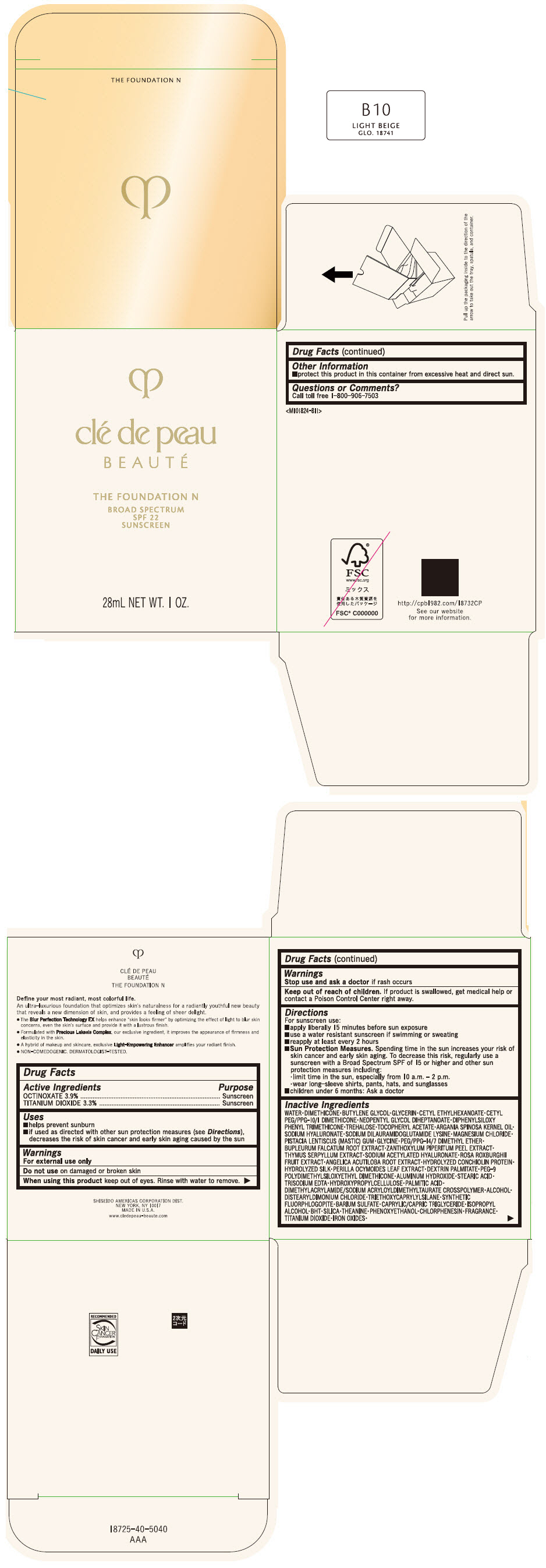
CLE DE PEAU BEAUTE THE FOUNDATION N B10 LIGHT BEIGE- octinoxate and titanium dioxide emulsion
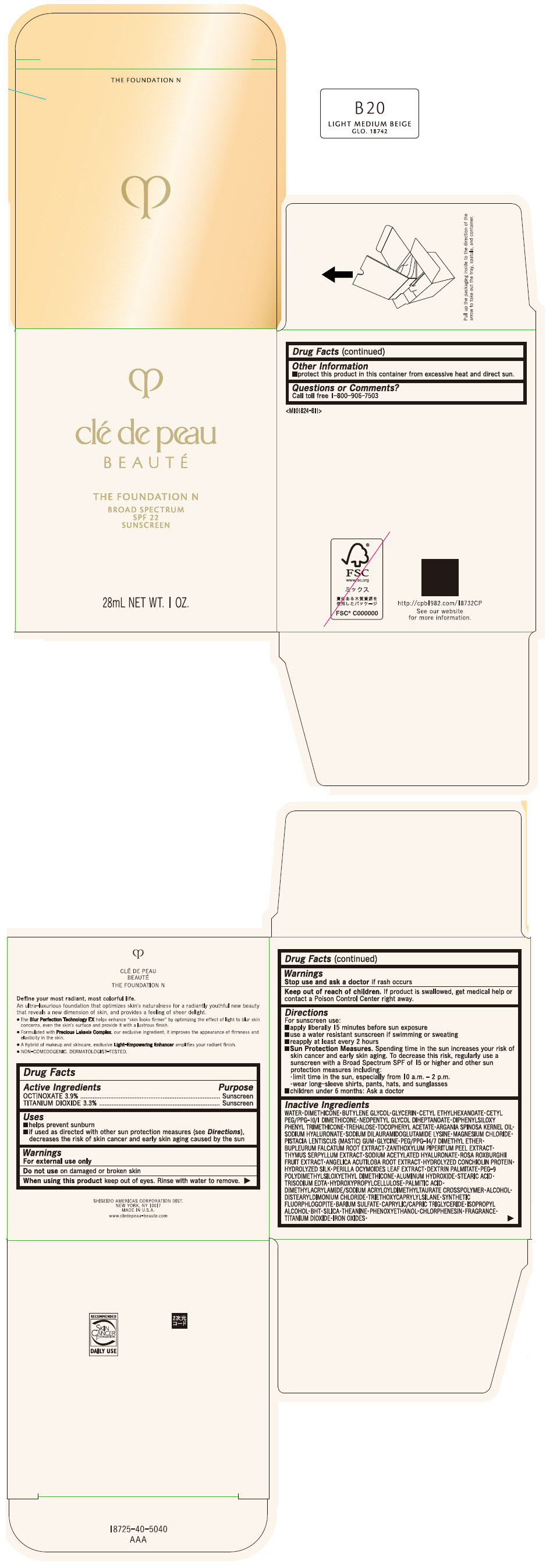
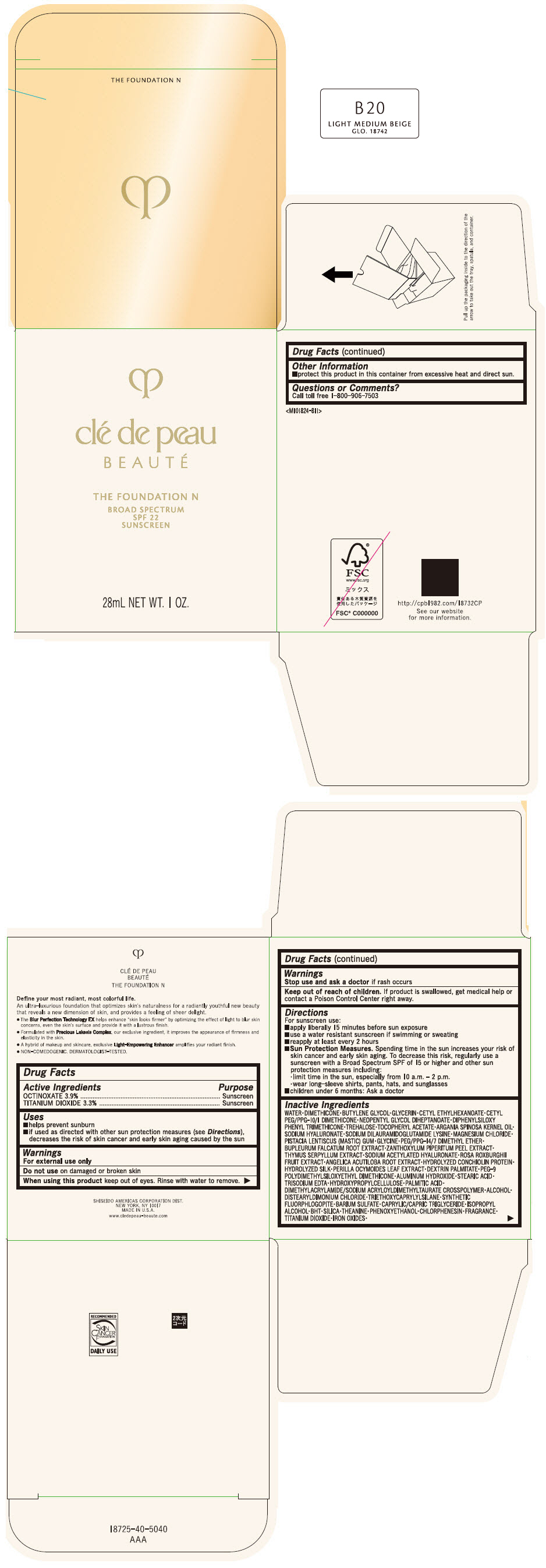
CLE DE PEAU BEAUTE THE FOUNDATION N B20 LIGHT MEDIUM BEIGE- octinoxate and titanium dioxide emulsion
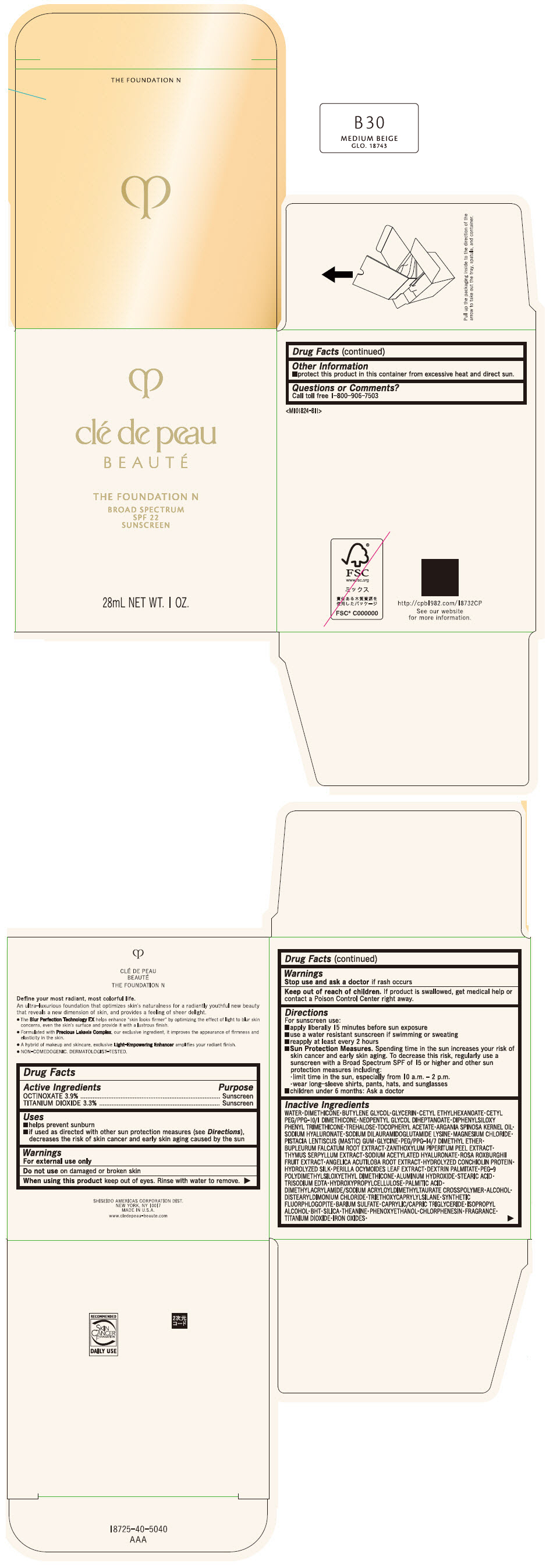
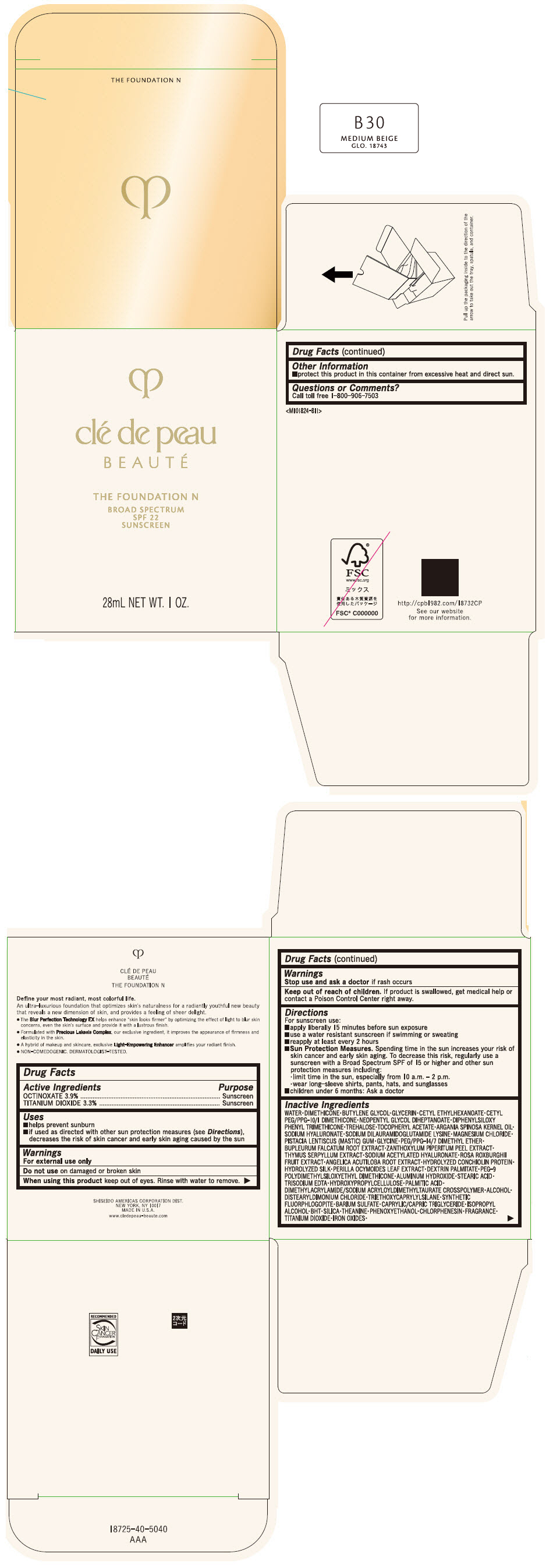
CLE DE PEAU BEAUTE THE FOUNDATION N B30 MEDIUM BEIGE- octinoxate and titanium dioxide emulsion
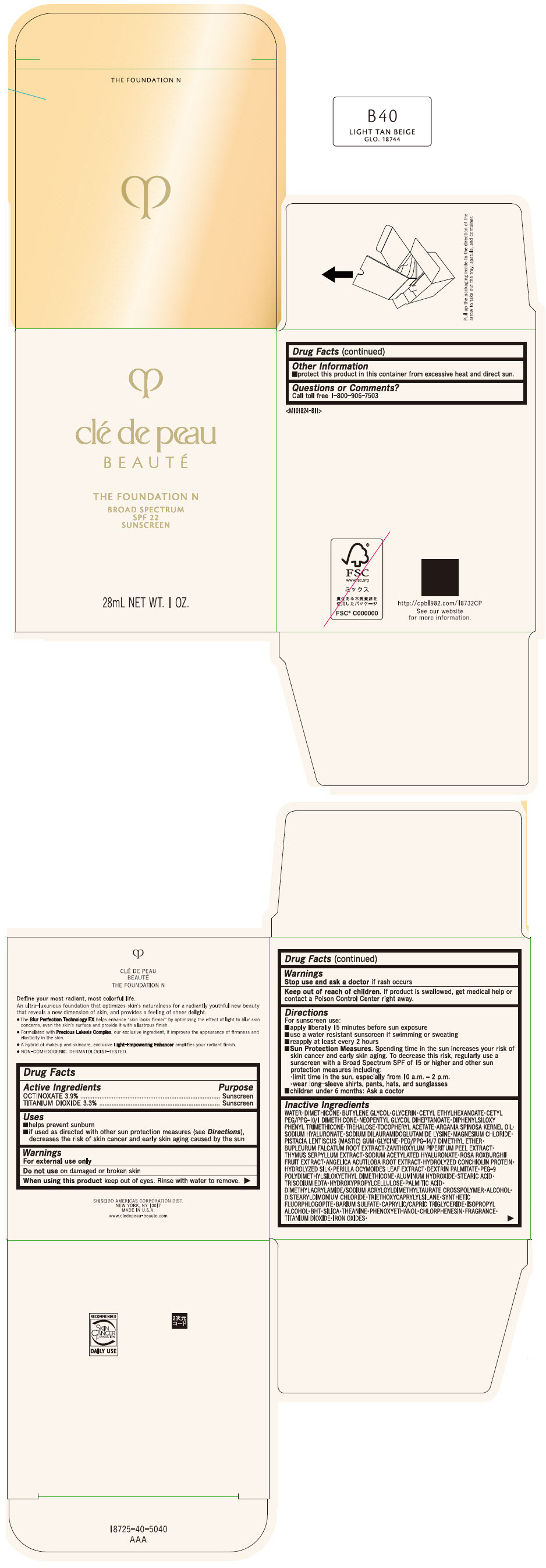
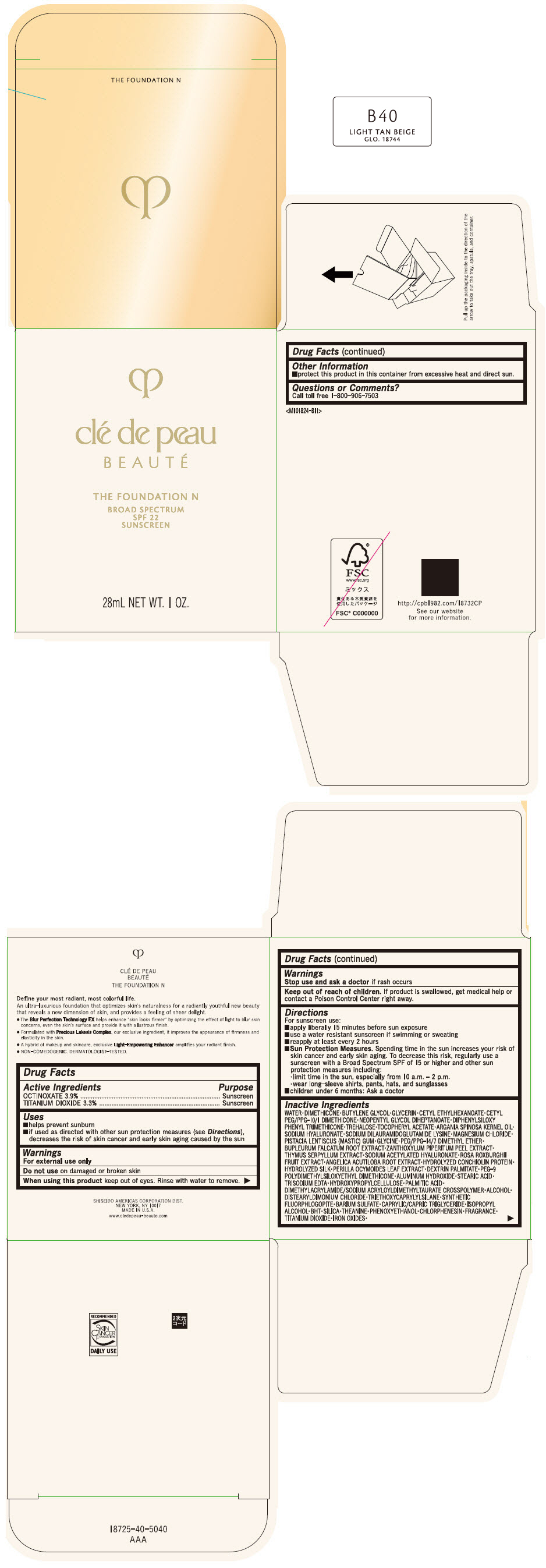
CLE DE PEAU BEAUTE THE FOUNDATION N B40 LIGHT TAN BEIGE- octinoxate and titanium dioxide emulsion
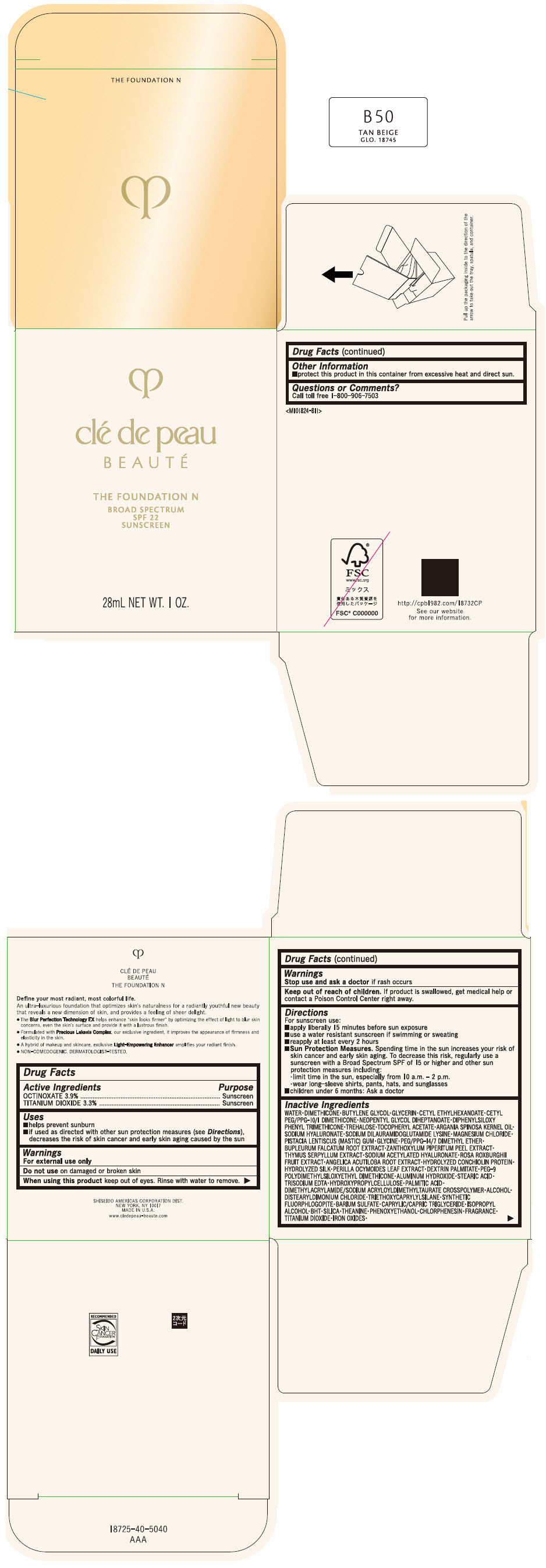
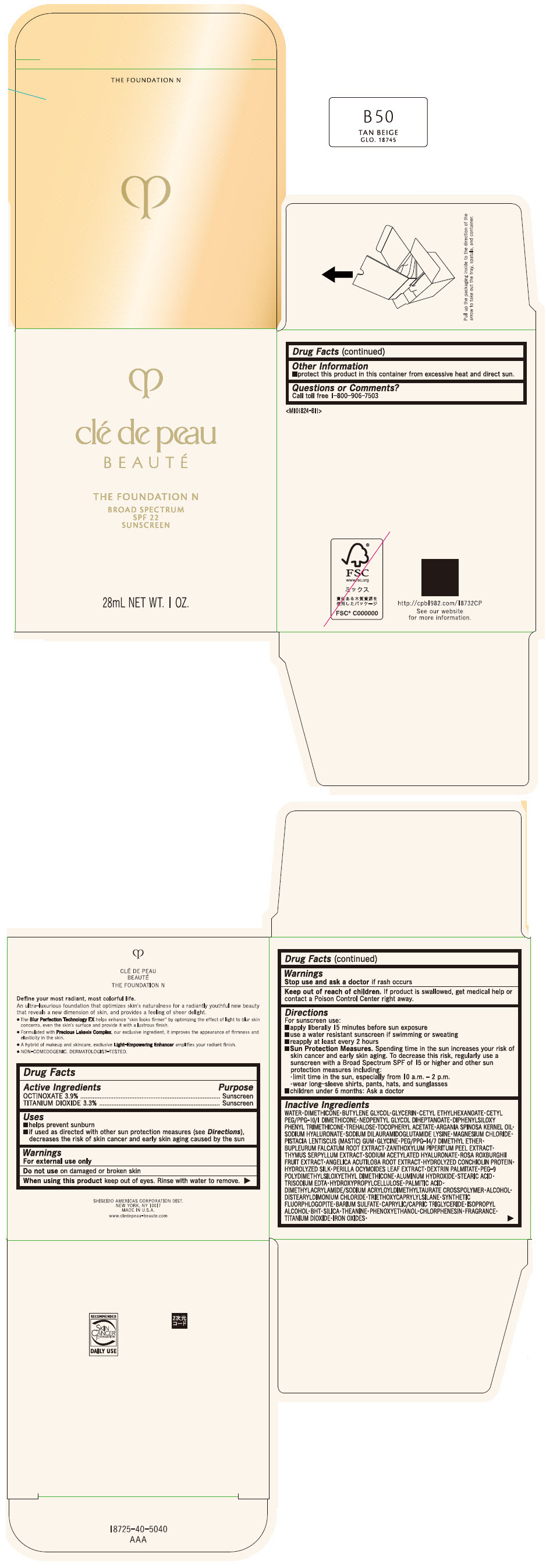
CLE DE PEAU BEAUTE THE FOUNDATION N B50 TAN BEIGE- octinoxate and titanium dioxide emulsion
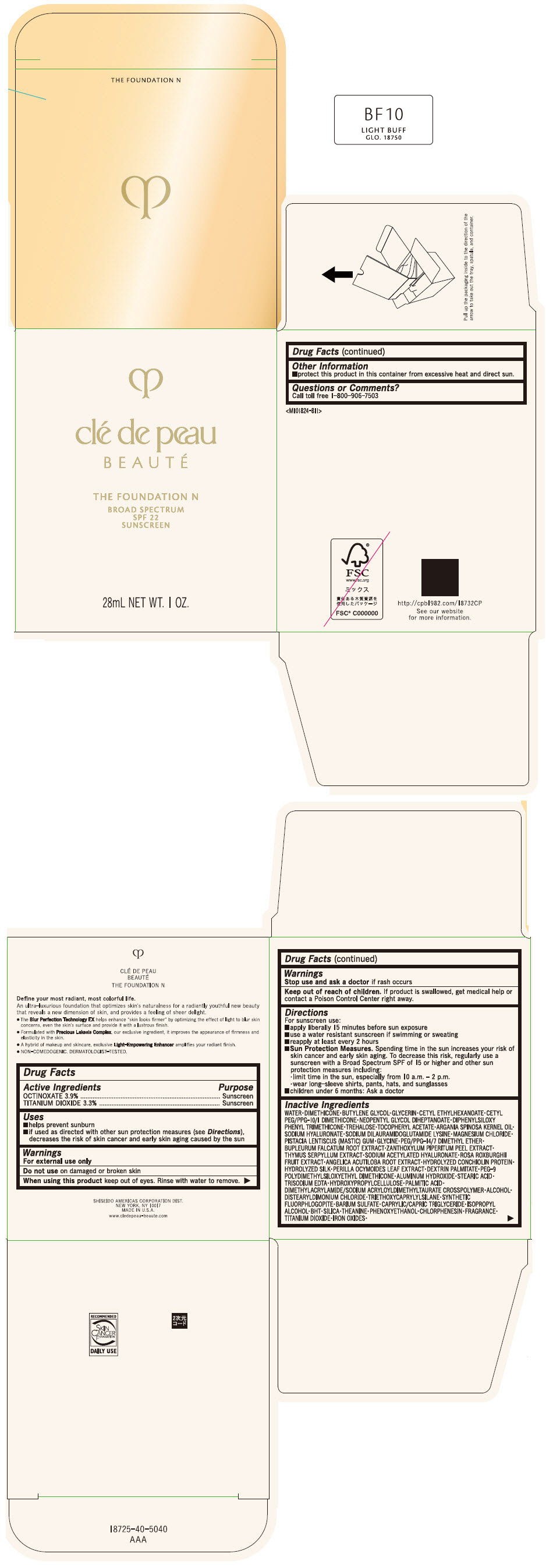
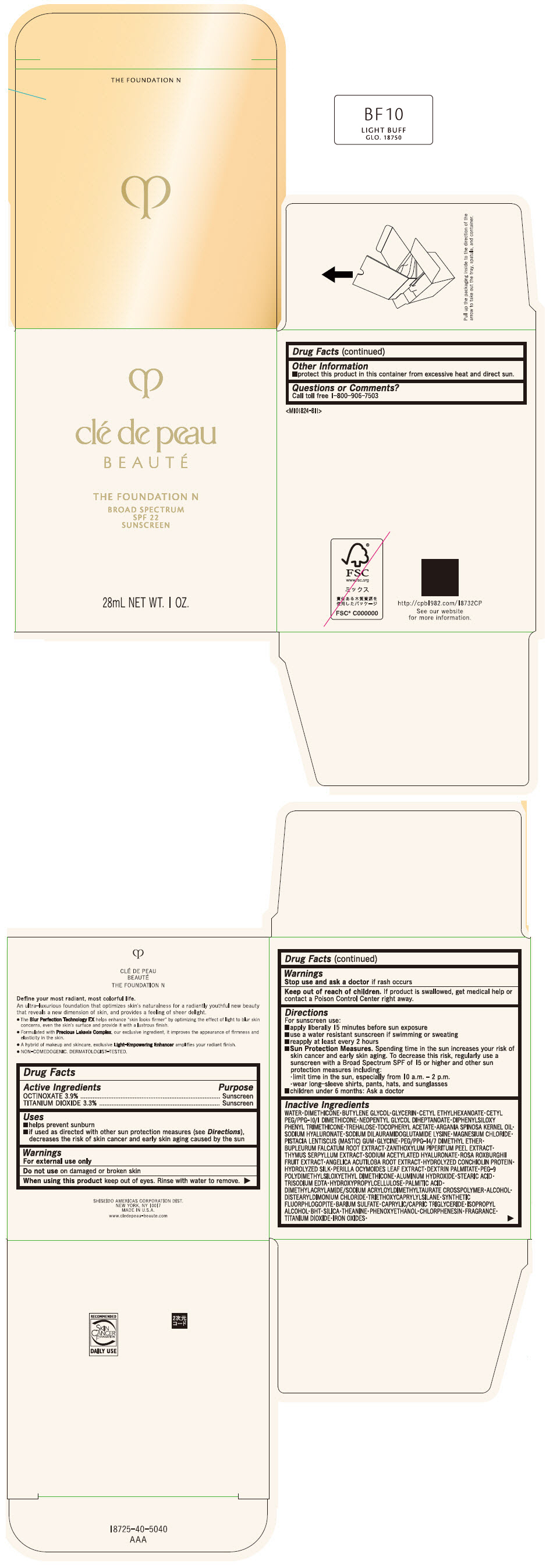
CLE DE PEAU BEAUTE THE FOUNDATION N BF10 LIGHT BUFF- octinoxate and titanium dioxide emulsion
-
NDC Code(s):
58411-839-50,
58411-840-50,
58411-841-50,
58411-842-50, view more58411-843-50, 58411-844-50, 58411-845-50, 58411-846-50, 58411-847-50, 58411-848-50, 58411-849-50, 58411-850-50, 58411-851-50, 58411-852-50, 58411-853-50
- Packager: SHISEIDO AMERICAS CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
-
Inactive Ingredients
WATER▪DIMETHICONE▪BUTYLENE GLYCOL▪GLYCERIN▪CETYL ETHYLHEXANOATE▪CETYL PEG/PPG-10/1 DIMETHICONE▪NEOPENTYL GLYCOL DIHEPTANOATE▪DIPHENYLSILOXY PHENYL TRIMETHICONE▪TREHALOSE▪TOCOPHERYL ACETATE▪ARGANIA SPINOSA KERNEL OIL▪SODIUM HYALURONATE▪SODIUM DILAURAMIDOGLUTAMIDE LYSINE▪MAGNESIUM CHLORIDE▪PISTACIA LENTISCUS (MASTIC) GUM▪GLYCINE▪PEG/PPG-14/7 DIMETHYL ETHER▪BUPLEURUM FALCATUM ROOT EXTRACT▪ZANTHOXYLUM PIPERITUM PEEL EXTRACT▪THYMUS SERPYLLUM EXTRACT▪SODIUM ACETYLATED HYALURONATE▪ROSA ROXBURGHII FRUIT EXTRACT▪ANGELICA ACUTILOBA ROOT EXTRACT▪HYDROLYZED CONCHIOLIN PROTEIN▪HYDROLYZED SILK▪PERILLA OCYMOIDES LEAF EXTRACT▪DEXTRIN PALMITATE▪PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE▪ALUMINUM HYDROXIDE▪STEARIC ACID▪TRISODIUM EDTA▪HYDROXYPROPYLCELLULOSE▪PALMITIC ACID▪DIMETHYLACRYLAMIDE/SODIUM ACRYLOYLDIMETHYLTAURATE CROSSPOLYMER▪ALCOHOL▪DISTEARYLDIMONIUM CHLORIDE▪TRIETHOXYCAPRYLYLSILANE▪SYNTHETIC FLUORPHLOGOPITE▪BARIUM SULFATE▪CAPRYLIC/CAPRIC TRIGLYCERIDE▪ISOPROPYL ALCOHOL▪BHT▪SILICA▪THEANINE▪PHENOXYETHANOL▪CHLOROPHENESIN▪FRAGRANCE▪TITANIUM DIOXIDE▪IRON OXIDES▪
- Other information
- Questions or Comments?
- PRINCIPAL DISPLAY PANEL - 28 mL Bottle Box - O50 Tan Ocher
- PRINCIPAL DISPLAY PANEL - 28 mL Bottle Box - I10 Very Light Ivory
- PRINCIPAL DISPLAY PANEL - 28 mL Bottle Box - O10 Light Ocher
- PRINCIPAL DISPLAY PANEL - 28 mL Bottle Box - O20 Light Medium Ocher
- PRINCIPAL DISPLAY PANEL - 28 mL Bottle Box - O30 Medium Ocher
- PRINCIPAL DISPLAY PANEL - 28 mL Bottle Box - O40 Light Tan Ocher
- PRINCIPAL DISPLAY PANEL - 28 mL Bottle Box - O60 Deep Tan Ocher
- PRINCIPAL DISPLAY PANEL - 28 mL Bottle Box - O80 Deep Ocher
- PRINCIPAL DISPLAY PANEL - 28 mL Bottle Box - O100 Rich Deep Ocher
- PRINCIPAL DISPLAY PANEL - 28 mL Bottle Box - B10 Light Beige
- PRINCIPAL DISPLAY PANEL - 28 mL Bottle Box - B20 Light Medium Beige
- PRINCIPAL DISPLAY PANEL - 28 mL Bottle Box - B30 Medium Beige
- PRINCIPAL DISPLAY PANEL - 28 mL Bottle Box - B40 Light Tan Beige
- PRINCIPAL DISPLAY PANEL - 28 mL Bottle Box - B50 Tan Beige
- PRINCIPAL DISPLAY PANEL - 28 mL Bottle Box - BF10 Light Buff
-
INGREDIENTS AND APPEARANCE
CLE DE PEAU BEAUTE THE FOUNDATION N O50 TAN OCHER
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-839 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1092 mg in 28 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 924 mg in 28 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE TRISODIUM (UNII: 420IP921MB) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) PALMITIC ACID (UNII: 2V16EO95H1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BARIUM SULFATE (UNII: 25BB7EKE2E) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) GLYCINE (UNII: TE7660XO1C) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ZANTHOXYLUM PIPERITUM FRUIT RIND (UNII: 728JDI7G4M) THYMUS SERPYLLUM WHOLE (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-839-50 1 in 1 BOX 04/01/2022 1 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/01/2022 CLE DE PEAU BEAUTE THE FOUNDATION N I10 VERY LIGHT IVORY
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-840 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1092 mg in 28 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 924 mg in 28 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE TRISODIUM (UNII: 420IP921MB) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) PALMITIC ACID (UNII: 2V16EO95H1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BARIUM SULFATE (UNII: 25BB7EKE2E) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) GLYCINE (UNII: TE7660XO1C) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ZANTHOXYLUM PIPERITUM FRUIT RIND (UNII: 728JDI7G4M) THYMUS SERPYLLUM WHOLE (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-840-50 1 in 1 BOX 04/01/2022 1 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/01/2022 CLE DE PEAU BEAUTE THE FOUNDATION N O10 LIGHT OCHER
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-841 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1092 mg in 28 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 924 mg in 28 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE TRISODIUM (UNII: 420IP921MB) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) PALMITIC ACID (UNII: 2V16EO95H1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BARIUM SULFATE (UNII: 25BB7EKE2E) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) GLYCINE (UNII: TE7660XO1C) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ZANTHOXYLUM PIPERITUM FRUIT RIND (UNII: 728JDI7G4M) THYMUS SERPYLLUM WHOLE (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-841-50 1 in 1 BOX 04/01/2022 1 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/01/2022 CLE DE PEAU BEAUTE THE FOUNDATION N O20 LIGHT MEDIUM OCHER
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-842 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1092 mg in 28 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 924 mg in 28 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE TRISODIUM (UNII: 420IP921MB) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) PALMITIC ACID (UNII: 2V16EO95H1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BARIUM SULFATE (UNII: 25BB7EKE2E) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) GLYCINE (UNII: TE7660XO1C) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ZANTHOXYLUM PIPERITUM FRUIT RIND (UNII: 728JDI7G4M) THYMUS SERPYLLUM WHOLE (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-842-50 1 in 1 BOX 04/01/2022 1 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/01/2022 CLE DE PEAU BEAUTE THE FOUNDATION N O30 MEDIUM OCHER
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-843 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1092 mg in 28 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 924 mg in 28 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE TRISODIUM (UNII: 420IP921MB) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) PALMITIC ACID (UNII: 2V16EO95H1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BARIUM SULFATE (UNII: 25BB7EKE2E) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) GLYCINE (UNII: TE7660XO1C) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ZANTHOXYLUM PIPERITUM FRUIT RIND (UNII: 728JDI7G4M) THYMUS SERPYLLUM WHOLE (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-843-50 1 in 1 BOX 04/01/2022 1 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/01/2022 CLE DE PEAU BEAUTE THE FOUNDATION N O40 LIGHT TAN OCHER
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-844 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1092 mg in 28 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 924 mg in 28 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE TRISODIUM (UNII: 420IP921MB) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) PALMITIC ACID (UNII: 2V16EO95H1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BARIUM SULFATE (UNII: 25BB7EKE2E) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) GLYCINE (UNII: TE7660XO1C) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ZANTHOXYLUM PIPERITUM FRUIT RIND (UNII: 728JDI7G4M) THYMUS SERPYLLUM WHOLE (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-844-50 1 in 1 BOX 04/01/2022 1 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/01/2022 CLE DE PEAU BEAUTE THE FOUNDATION N O60 DEEP TAN OCHER
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-845 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1092 mg in 28 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 924 mg in 28 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE TRISODIUM (UNII: 420IP921MB) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) PALMITIC ACID (UNII: 2V16EO95H1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BARIUM SULFATE (UNII: 25BB7EKE2E) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) GLYCINE (UNII: TE7660XO1C) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ZANTHOXYLUM PIPERITUM FRUIT RIND (UNII: 728JDI7G4M) THYMUS SERPYLLUM WHOLE (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-845-50 1 in 1 BOX 04/01/2022 1 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/01/2022 CLE DE PEAU BEAUTE THE FOUNDATION N O80 DEEP OCHER
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-846 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1092 mg in 28 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 924 mg in 28 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE TRISODIUM (UNII: 420IP921MB) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) PALMITIC ACID (UNII: 2V16EO95H1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BARIUM SULFATE (UNII: 25BB7EKE2E) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) GLYCINE (UNII: TE7660XO1C) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ZANTHOXYLUM PIPERITUM FRUIT RIND (UNII: 728JDI7G4M) THYMUS SERPYLLUM WHOLE (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-846-50 1 in 1 BOX 04/01/2022 1 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/01/2022 CLE DE PEAU BEAUTE THE FOUNDATION N O100 RICH DEEP OCHER
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-847 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1092 mg in 28 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 924 mg in 28 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE TRISODIUM (UNII: 420IP921MB) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) PALMITIC ACID (UNII: 2V16EO95H1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BARIUM SULFATE (UNII: 25BB7EKE2E) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) GLYCINE (UNII: TE7660XO1C) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ZANTHOXYLUM PIPERITUM FRUIT RIND (UNII: 728JDI7G4M) THYMUS SERPYLLUM WHOLE (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-847-50 1 in 1 BOX 04/01/2022 1 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/01/2022 CLE DE PEAU BEAUTE THE FOUNDATION N B10 LIGHT BEIGE
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-848 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1092 mg in 28 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 924 mg in 28 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE TRISODIUM (UNII: 420IP921MB) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) PALMITIC ACID (UNII: 2V16EO95H1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BARIUM SULFATE (UNII: 25BB7EKE2E) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) GLYCINE (UNII: TE7660XO1C) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ZANTHOXYLUM PIPERITUM FRUIT RIND (UNII: 728JDI7G4M) THYMUS SERPYLLUM WHOLE (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-848-50 1 in 1 BOX 04/01/2022 1 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/01/2022 CLE DE PEAU BEAUTE THE FOUNDATION N B20 LIGHT MEDIUM BEIGE
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-849 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1092 mg in 28 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 924 mg in 28 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE TRISODIUM (UNII: 420IP921MB) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) PALMITIC ACID (UNII: 2V16EO95H1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BARIUM SULFATE (UNII: 25BB7EKE2E) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) GLYCINE (UNII: TE7660XO1C) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ZANTHOXYLUM PIPERITUM FRUIT RIND (UNII: 728JDI7G4M) THYMUS SERPYLLUM WHOLE (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-849-50 1 in 1 BOX 04/01/2022 1 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/01/2022 CLE DE PEAU BEAUTE THE FOUNDATION N B30 MEDIUM BEIGE
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-850 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1092 mg in 28 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 924 mg in 28 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE TRISODIUM (UNII: 420IP921MB) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) PALMITIC ACID (UNII: 2V16EO95H1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BARIUM SULFATE (UNII: 25BB7EKE2E) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) GLYCINE (UNII: TE7660XO1C) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ZANTHOXYLUM PIPERITUM FRUIT RIND (UNII: 728JDI7G4M) THYMUS SERPYLLUM WHOLE (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-850-50 1 in 1 BOX 04/01/2022 1 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/01/2022 CLE DE PEAU BEAUTE THE FOUNDATION N B40 LIGHT TAN BEIGE
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-851 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1092 mg in 28 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 924 mg in 28 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE TRISODIUM (UNII: 420IP921MB) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) PALMITIC ACID (UNII: 2V16EO95H1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BARIUM SULFATE (UNII: 25BB7EKE2E) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) GLYCINE (UNII: TE7660XO1C) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ZANTHOXYLUM PIPERITUM FRUIT RIND (UNII: 728JDI7G4M) THYMUS SERPYLLUM WHOLE (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-851-50 1 in 1 BOX 04/01/2022 1 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/01/2022 CLE DE PEAU BEAUTE THE FOUNDATION N B50 TAN BEIGE
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-852 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1092 mg in 28 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 924 mg in 28 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE TRISODIUM (UNII: 420IP921MB) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) PALMITIC ACID (UNII: 2V16EO95H1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BARIUM SULFATE (UNII: 25BB7EKE2E) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) GLYCINE (UNII: TE7660XO1C) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ZANTHOXYLUM PIPERITUM FRUIT RIND (UNII: 728JDI7G4M) THYMUS SERPYLLUM WHOLE (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-852-50 1 in 1 BOX 04/01/2022 1 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/01/2022 CLE DE PEAU BEAUTE THE FOUNDATION N BF10 LIGHT BUFF
octinoxate and titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58411-853 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1092 mg in 28 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 924 mg in 28 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) FERRIC OXIDE RED (UNII: 1K09F3G675) DIPHENYLSILOXY PHENYL TRIMETHICONE (UNII: I445L28B12) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE TRISODIUM (UNII: 420IP921MB) ARGAN OIL (UNII: 4V59G5UW9X) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) PALMITIC ACID (UNII: 2V16EO95H1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TREHALOSE (UNII: B8WCK70T7I) ALCOHOL (UNII: 3K9958V90M) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) SODIUM DILAURAMIDOGLUTAMIDE LYSINE (UNII: MNJ7VPT2R5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BARIUM SULFATE (UNII: 25BB7EKE2E) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PISTACIA LENTISCUS RESIN (UNII: 7446H202QW) GLYCINE (UNII: TE7660XO1C) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) THEANINE (UNII: 8021PR16QO) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ZANTHOXYLUM PIPERITUM FRUIT RIND (UNII: 728JDI7G4M) THYMUS SERPYLLUM WHOLE (UNII: 86H4S6K51N) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) PERILLA FRUTESCENS LEAF (UNII: T4L5881Y68) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58411-853-50 1 in 1 BOX 04/01/2022 1 28 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 04/01/2022 Labeler - SHISEIDO AMERICAS CORPORATION (193691821)