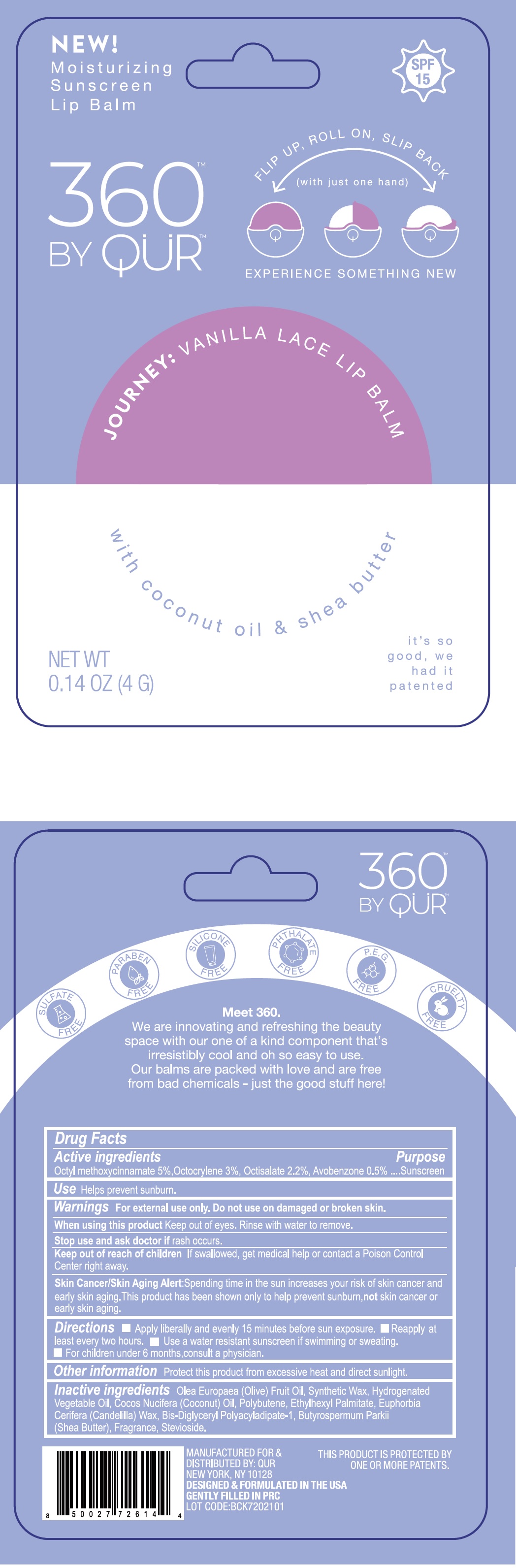
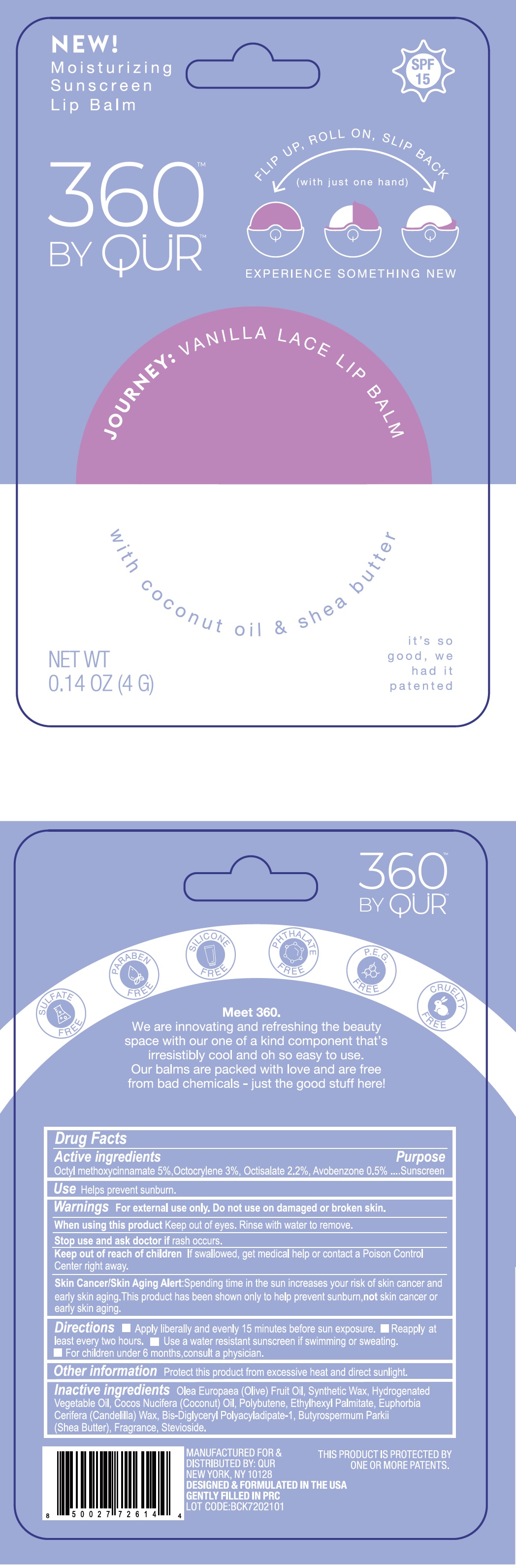
Label: 360 BY QUR VANILLA LACE LIP BALM- octinoxate, octocrylene, octisalate, avobenzone stick
- NDC Code(s): 81761-115-01
- Packager: Qur, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Use
-
Warnings
For external use only.
Keep out of the reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Spending time in the sun increase your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging. Skin Cancer/Skin Aging Alert:
- Directions
- Other information
- Inactive ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
360 BY QUR VANILLA LACE LIP BALM
octinoxate, octocrylene, octisalate, avobenzone stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81761-115 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 50 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 30 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 22 mg in 1 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 5 mg in 1 g Inactive Ingredients Ingredient Name Strength OLIVE OIL (UNII: 6UYK2W1W1E) COCONUT OIL (UNII: Q9L0O73W7L) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) ETHYLHEXYL PALMITATE (UNII: 2865993309) CANDELILLA WAX (UNII: WL0328HX19) BIS-DIGLYCERYL POLYACYLADIPATE-1 (UNII: F6ZM55051M) SHEA BUTTER (UNII: K49155WL9Y) STEVIOSIDE (UNII: 0YON5MXJ9P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81761-115-01 4 g in 1 CASE; Type 0: Not a Combination Product 02/24/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/24/2021 Labeler - Qur, Inc (072265258)