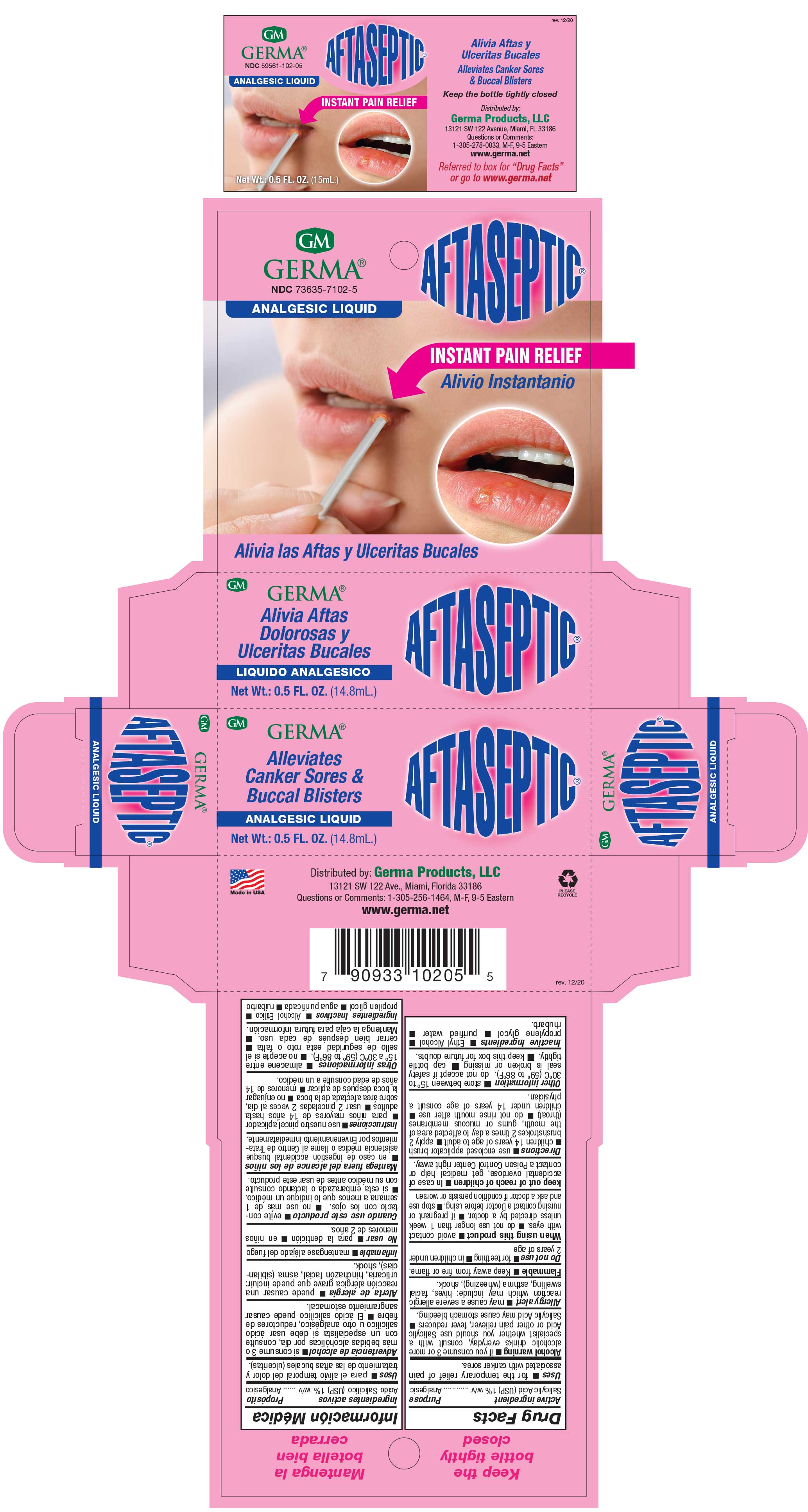
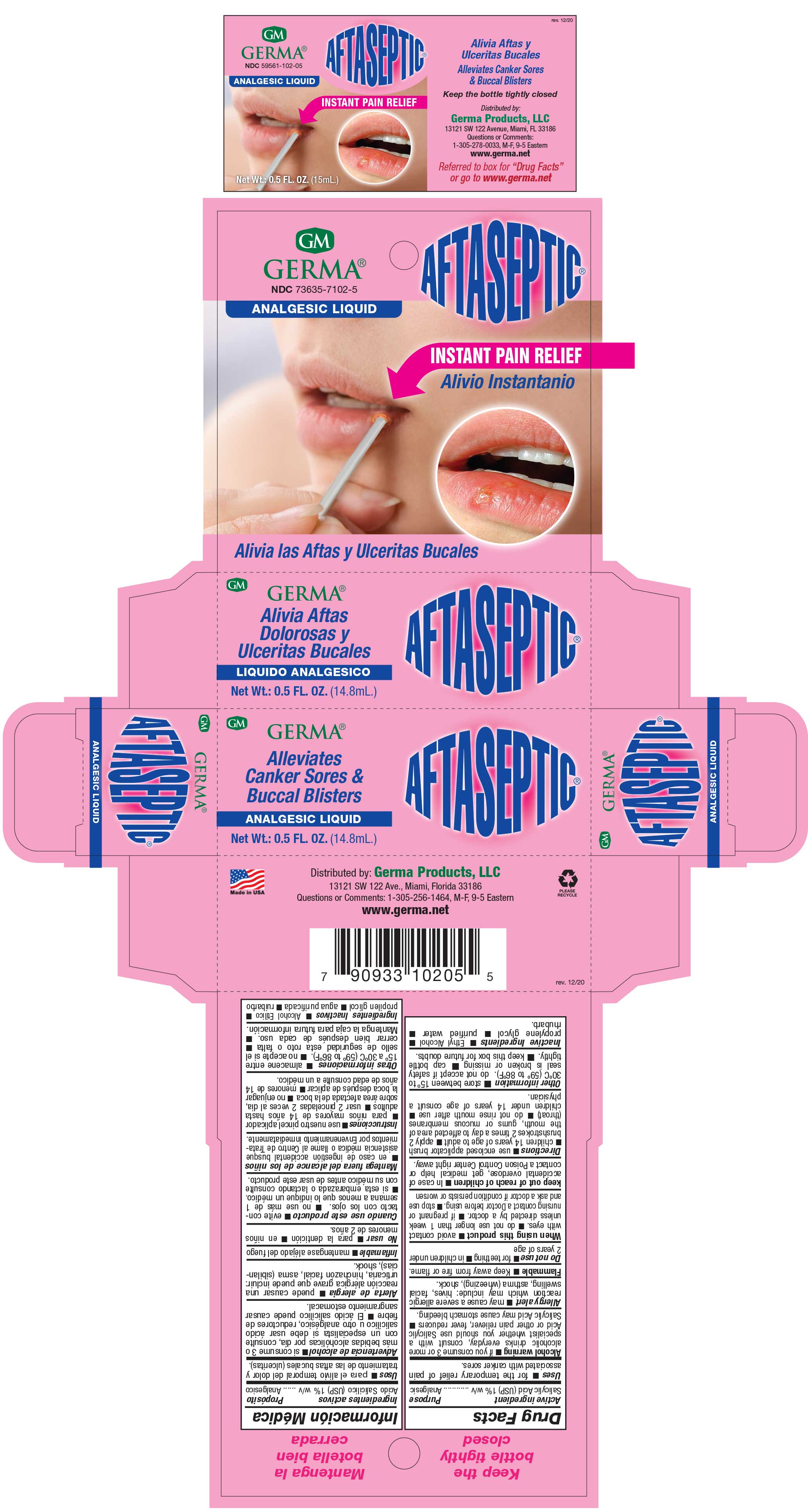
Label: AFTASEPTIC INSTANT PAIN RELIEF- salisylic acid 1% liquid
- NDC Code(s): 73635-7102-5
- Packager: Germa Products, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- INDICATIONS & USAGE
-
Warnings
Alcohol warning: if you consume 3 or more alcoholic drinks everyday, consult with a specialist whether you should use Salicylic Acid or other pain reliever, fever reducers n Salicylic Acid may cause stomach bleeding.
Allergy alert: may cause a severe allergic reaction which may include: hives, facial swelling, asthma (wheezing), shock.
-
Warnings
Flammable n Keep away from fire or flame. Do not use for teething or in children under 2 years of age
When using this product n avoid contact with eyes. Do not use longer than 1 week unless directed by a doctor. If pregnant or nursing contact a Doctor before using. Stop use and ask a doctor if condition persists or worsen
keep out of reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away. - Other Information
- Directions
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AFTASEPTIC INSTANT PAIN RELIEF
salisylic acid 1% liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73635-7102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALCOHOL (UNII: 3K9958V90M) RHUBARB (UNII: G280W4MW6E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73635-7102-5 1 in 1 BOX 03/29/2019 1 14.8 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 03/29/2019 Labeler - Germa Products, LLC (116626935)