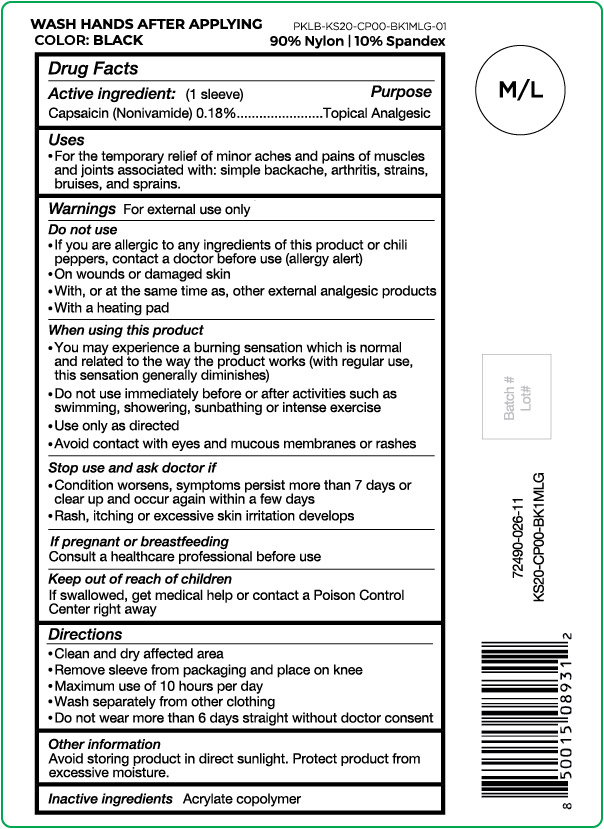
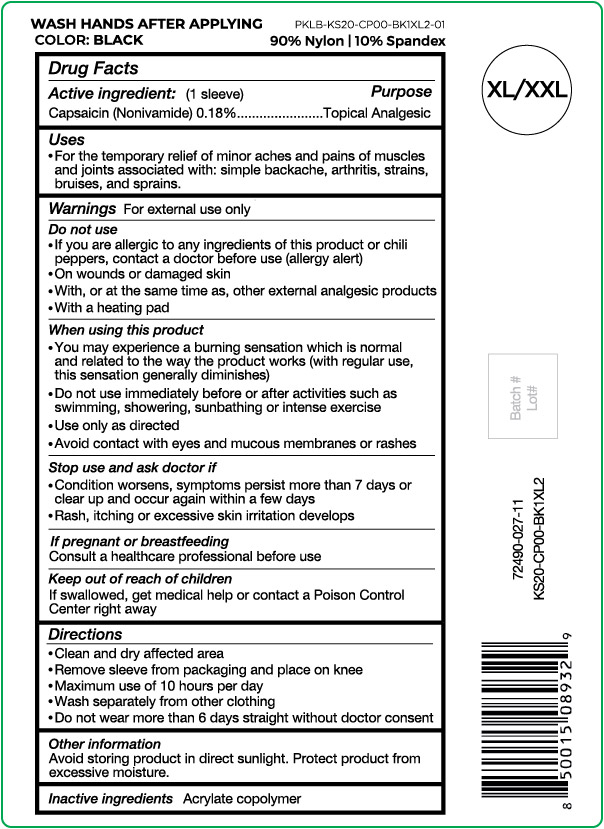
Label: NUFABRX KNEE SLEEVE- capsaicin cloth
- NDC Code(s): 72490-025-11, 72490-026-11, 72490-027-11
- Packager: Nufabrx LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
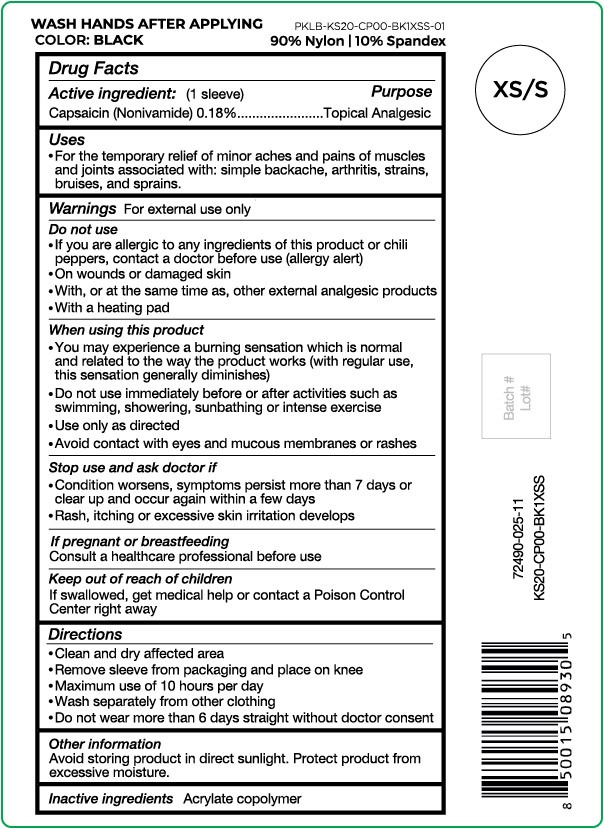
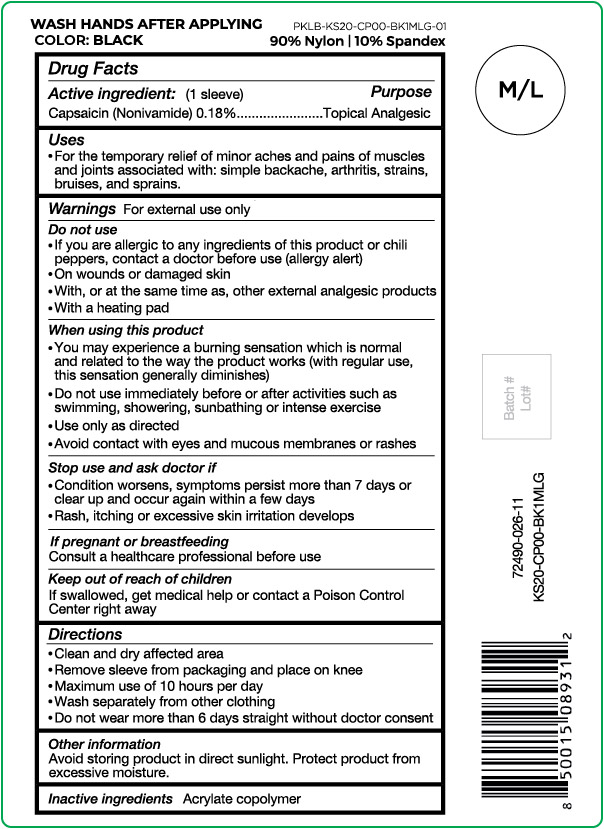
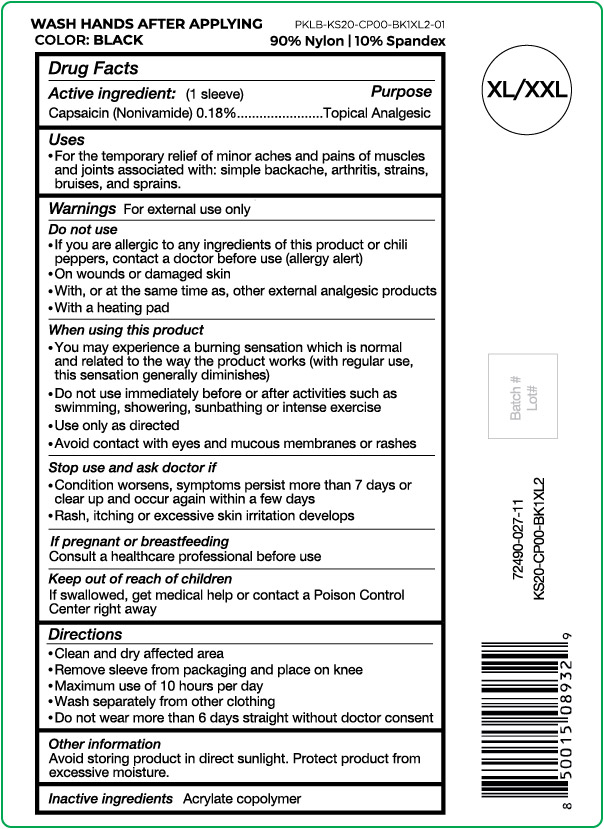
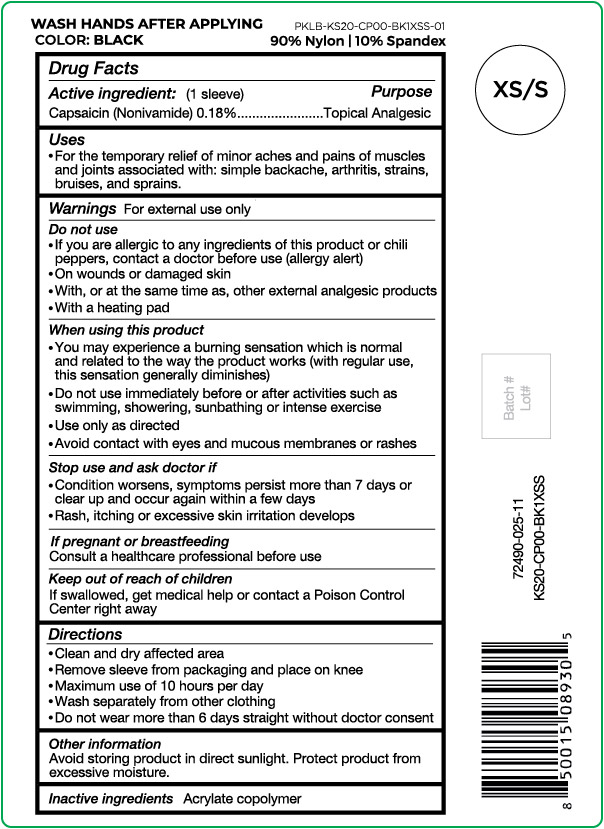
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- If you are allergic to any ingredients of this product or chili peppers, contact a doctor before use (allergy alert)
- On wounds or damaged skin
- With, or at the same time as, other external analgesic products
- With a heating pad
When using this product
- You may experience a burning sensation which is normal and related to the way the product works (with regular use, this sensation generally diminishes)
- Do not use immediately before or after activities such as swimming, showering, sunbathing or intense exercise
- Use only as directed
- Avoid contact with eyes and mucous membranes or rashes
- Directions
- Other information
- Inactive ingredients
- Principal Display and Drug Facts
-
INGREDIENTS AND APPEARANCE
NUFABRX KNEE SLEEVE
capsaicin clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72490-026 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.18 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) Product Characteristics Color black (Size M/L) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72490-026-11 1 g in 1 POUCH; Type 0: Not a Combination Product 03/29/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/29/2021 NUFABRX KNEE SLEEVE
capsaicin clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72490-027 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.18 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) Product Characteristics Color black (Size XL/XXL) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72490-027-11 1 g in 1 POUCH; Type 0: Not a Combination Product 03/29/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/29/2021 NUFABRX KNEE SLEEVE
capsaicin clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72490-025 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.18 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) Product Characteristics Color black (Size XS/S) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72490-025-11 1 g in 1 POUCH; Type 0: Not a Combination Product 03/29/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/29/2021 Labeler - Nufabrx LLC (119110776) Registrant - Biotech Research Group (099152813)