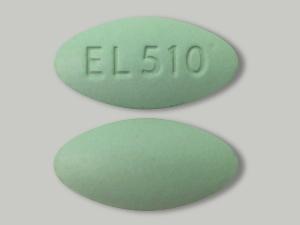
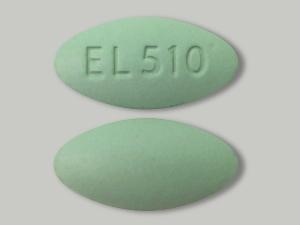
Label: NICAZELFORTE- azerizin, pyridoxine, zinc, copper, folic acid tablet
- NHRIC Code(s): 42783-510-60, 42783-510-04
- Packager: Elorac, Inc
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated December 8, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
NicAzel FORTE (Azerizin, zinc, pyridoxine, copper, folic acid)
Rx Dietary Supplement
DESCRIPTION
NicAzel FORTE Tablets is a uniquely formulated dietary supplement for oral administration. Each green-colored, oval-shaped tablet is imprinted on one side with “EL510” and contains Azerizin®, a proprietary blend of natural ingredients which combine demonstrated anti-inflammatory and anti-microbial properties, along with inhibiting effects on sebum production.
- Ingredients:
- Other Ingredients:
-
CLINICAL PHARMACOLOGY
Nicotinamide is a water-soluble component of the vitamin B complex group. In vivo, nicotinamide is incorporated into nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). NAD and NADP function as coenzymes in a wide variety of enzymatic oxidation-reduction reactions essential for tissue respiration, lipid metabolism, and glycogenolysis.
Nicotinamide has demonstrated anti-inflammatory actions that may be of benefit in patients with acne including, but not limited to, suppression of antigen-induced lymphocytic transformation and inhibition of 3´, 5´-cyclic AMP phosphodiesterase. Nicotinamide has been demonstrated to block the inflammatory actions of iodides known to precipitate or exacerbate acne.
Nicotinamide lacks the vasodilator, gastrointestinal, hepatic, and hypolipemic actions of nicotinic acid or niacin. As such, nicotinamide has not been shown to produce the flushing, itching, and burning sensations of the skin, as is commonly seen when large doses of nicotinic acid or niacin are administered orally.
Azelaic acid is a dietary constituent (e.g., in whole grain cereals and animal products) that has been shown to possess antimicrobial activity against Propionibacterium acnes and Staphylococcus epidermidis. The antimicrobial action may be attributable to inhibition of microbial cellular protein synthesis. A normalization of keratinization leading to an anticomedonal effect of azelaic acid may also contribute to its topical use for inflammatory acne vulgaris and rosacea.
Quercetin is a polyphenolic substance and a member of the class of flavonoids called flavonols. Quercetin is widely distributed in the plant kingdom, with onions, apples, red wine and green tea especially rich sources of the agent. Quercetin is an antioxidant which inhibits lipid peroxidation. Its potent anti-inflammatory activity is at least in part accounted for by its inhibition of degranulation of mast cells, basophils and neutrophils, coupled with its modulation of the activity of the inflammatory mediator NF-kappa B. Quercetin has been reported to reduce inflammation in both acne and rosacea.
Curcumin is a polyphenolic substance principally found in the spices turmeric and ginger. Curcumin has been demonstrated to have antioxidant, anti-inflammatory, and antimicrobial activities, and has been clinically most widely evaluated for its purported anti-cancer and cancer chemopreventive properties. Curcumin has been widely employed in the management of acne vulgaris and rosacea, based on its significant inhibition of Propionibacterium acnes and its potent anti-inflammatory actions, which include inhibition of cyclooxygenase (COX) and lipoxygenase (LOX), reduction of the release of reactive oxygen species (ROS) by stimulated neutrophils, and its inhibition of the activation of proinflammatory cytokines.
Pyridoxine or Vitamin B6 is a water soluble component of the vitamin B complex group, which in the form of the coenzyme pyridoxal 5´-phosphate is involved in a wide range of biochemical reactions, and is present in a wide variety of food sources including meat, poultry, fish, eggs, starchy vegetables, and non-citrus fruits. Pyridoxine plays an active role in the immune system, and supplementation to normal levels in individuals with even a marginal deficiency generally restores immune functions due to deficiency. Pyridoxine supplementation has been found helpful in the management of acne, particularly for women with premenstrual flares of acne.
Zinc has been shown to inhibit the inflammatory polymorphonuclear leukocyte chemotaxis in acne patients. Zinc has also demonstrated an inhibitory effect on the lipase of the three Propionibacterium species found in human pilosebaceous follicles. Patients with acne have been shown to have significantly lower serum zinc levels than matched healthy controls.
Copper is an essential trace mineral in human nutrition. Although rare, copper deficiency has been induced by supplemental zinc therapy.
Folic acid serves as an essential cofactor of the biosynthesis of thymidine and purine nucleotides required for normal cellular DNA synthesis. Deficiencies of folic acid have been demonstrated to occur in some cutaneous disorders.
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
- USE
- WARNINGS
- PRECAUTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- DOSAGE & ADMINISTRATION
-
Principal Display Panel with Package Insert (Booklet Label):
42783-510-04
NicAzel®FORTE
(Azerizin®, zinc, pyridoxine, copper, folic acid)Prescription Dietary Supplement
4 Tablets
42783-510-60
NicAzel®FORTE
(Azerizin®, zinc, pyridoxine, copper, folic acid)Prescription Dietary Supplement
60 Tablets
-
INGREDIENTS AND APPEARANCE
NICAZELFORTE
azerizin, pyridoxine, zinc, copper, folic acid tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:42783-510 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 12 mg PYRIDOXINE (UNII: KV2JZ1BI6Z) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 8 mg CUPRIC OXIDE (UNII: V1XJQ704R4) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 2 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 500 ug Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) POLYVINYL ALCOHOL (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:42783-510-60 60 in 1 BOTTLE 2 NHRIC:42783-510-04 4 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 12/01/2013 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 19 mm scoring 1 imprint Labeler - Elorac, Inc (832590009)