Label: ELURA- capromorelin solution
- NDC Code(s): 58198-5545-1
- Packager: Elanco US Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated November 20, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
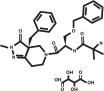
ELURA (capromorelin oral solution) is a colorless to yellow or orange, clear liquid. Each milliliter of ELURA contains 20 mg of capromorelin tartrate. The empirical formula is C28H35N5O4·C4H6O6 and the molecular weight 655.70. The chemical name is 2-aminoN-[2-(3aR-benzyl-2-methyl-3-oxo-2,3,3a,4,6,7-hexahydro-pyrazolo[4,3-c]pyridine-5-yl)-1R-benzyloxymethyl-2-oxo-ethyl]-isobutyramide L-tartrate. The chemical structure of capromorelin tartrate is:
- INDICATION:
-
DOSAGE AND ADMINISTRATION:
Administer ELURA orally at a dose of 2 mg/kg (0.9 mg/lb) or 0.1 mL/kg (0.045 mL/lb) body weight once daily.
To administer ELURA:
- Remove the cap, insert the dosing syringe, invert the bottle, withdraw the appropriate amount of solution.
- Return the bottle to the upright position, remove syringe, replace the cap.
- Administer the solution into the cat's mouth.
- Rinse the syringe and plunger with water and leave apart to dry.
If the cat is routinely fed meals, offer food 30 minutes after administering the dose.
If the cat vomits within 15 minutes or only receives a partial dose, then the dose may be re-administered.
- CONTRAINDICATIONS:
-
WARNINGS:
Not for use in humans. Keep this and all medications out of reach of children and pets. Consult a physician in case of accidental ingestion by humans.
For oral use in cats only.
Do not use in cats with hypersomatotropism (acromegaly).
ELURA may increase serum glucose for several hours after dosing (see Animal Safety and Clinical Pharmacology). Use in cats with current or historical diabetes mellitus has not been evaluated and use may not be appropriate.
-
PRECAUTIONS:
Use with caution in cats that may have cardiac disease or severe dehydration. ELURA causes transient decreases in heart rate and blood pressure up to 4 hours following dose administration. Some cats may exhibit clinical signs of bradycardia or hypotension following administration of ELURA. (See Adverse Reactions and Animal Safety).
Use with caution in cats with hepatic dysfunction. Capromorelin is metabolized in the liver in humans and dogs and similar metabolism is expected in the cat. The safe use of ELURA has not been evaluated in cats younger than 5 months old.
The safe use of ELURA has not been evaluated in cats that are pregnant, lactating, or intended for breeding.
-
ADVERSE REACTIONS:
Safety was evaluated in a 56-day field effectiveness study in 176 client-owned cats (118 administered ELURA, 58 administered vehicle control) that received at least one dose.
Cats enrolled had ≥5% unintended weight loss and a history of chronic kidney disease (CKD). Cats had a mean age of 15 years and at enrollment 11.4% of the cats were in Stage 1 CKD, 66.5% were in Stage 2, 21.0% were in Stage 3, and 1.1% were in Stage 4. Cats enrolled in the study had a variety of comorbid conditions: dental disease (88.1%), moderate or severe muscle loss (43.2%), heart murmur (28.4%), history of vomiting or underlying gastrointestinal disease (28.4%), hyperthyroidism (13.6%) and hypertension (9.7%).
Table 1: Adverse Reactions in the Field Effectiveness Study Note: If an animal experienced the same event more than once, only the first occurrence was tabulated.
a Behavior change included hiding from the owner (8 ELURA, 1 vehicle control); owner reported difficulty administering medication (7 ELURA, 1 vehicle control); and redirected aggression to another household cat (2 ELURA, 1 vehicle control).
b Two ELURA and 1 vehicle control cat increased by two CKD stages; 8 ELURA and 2 vehicle control cats increased one CKD stage. It could not be determined if the progressive renal disease was the natural course of the pre-existing disease or treatment related.
Adverse Reaction ELURA (n=118) Vehicle Control (n=58) Vomiting 35 (29.6%) 13 (22.4%) Hypersalivation 25 (21.2%) 0 (0.0%) Inappetence 22 (18.6%) 7 (12.0%) Behavior Change a 17 (14.4%) 3 (5.2%) Lethargy 16 (13.6%) 6 (10.3%) Anemia 11 (9.3%) 1 (1.7%) Dehydration 11 (9.3%) 2 (3.4%) Stage of CKD Increased b 10 (8.5%) 3 (5.2%) Diarrhea 9 (7.6%) 2 (3.4%) Urinary Tract Infection 8 (6.8%) 2 (3.4%) Hyperglycemia 8 (6.8%) 2 (3.4%) Upper Respiratory Infection 7 (5.9%) 1 (1.7%) Hypercalcemia 7 (5.9%) 0 (0.0%) Facial Skin Lesion 6 (5.1%) 3 (5.2%) Hyperkalemia 5 (4.2%) 0 (0.0%) Ataxia 4 (3.4%) 0 (0.0%) Diabetes Mellitus 1 (0.8%) 0 (0.0%) Congestive Heart Failure 1 (0.8%) 0 (0.0%) Hypersalivation was generally associated with dosing and resolved within a few minutes.
Nine cats (8 ELURA and 1 vehicle control) either died or were euthanized during or shortly after the study. Six ELURA cats were euthanized or died from decompensated CKD. One ELURA cat was euthanized after study withdrawal on Day 33 for declining quality of life and recent identification of a new mass. One ELURA cat acutely declined and was euthanized for findings of nodules in both kidneys and diagnosis of sarcoma. The vehicle control cat was euthanized for acute onset of right hindlimb paresis and suspected embolic event. Two additional cats were diagnosed with neoplasia during the study (one ELURA cat with unspecified soft tissue sarcoma and one control cat with mammary adenocarcinoma) but completed the study. In voluntary post-approval reporting for extra-label use of a capromorelin product for dogs, the following adverse events have been reported in cats (listed in decreasing order of reporting frequency): bradycardia, lethargy, hypersalivation, hypotension, behavior change, and vomiting.
To report suspected adverse events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Elanco US, Inc. at 1-888-545-5973.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
-
INFORMATION FOR CAT OWNERS:
Owners should be advised that ELURA mimics the action of a naturally-occurring hormone called ghrelin. Ghrelin influences many systems in the body. ELURA may also affect these systems. Owners should monitor for changes in: thirst or water intake; lethargy or weakness; digestive issues (vomiting, diarrhea, drooling, decreased appetite); or behaviors.
-
CLINICAL PHARMACOLOGY:
Mechanism of Action
ELURA is a selective ghrelin receptor agonist. The ghrelin receptor is found in many tissues in various species and may have effects in the central nervous system, gastrointestinal tract, cardiovascular system and energy homeostasis. ELURA binds to receptors in the hypothalamus to stimulate appetite and in the pituitary to stimulate secretion of growth hormone (GH). Increased GH stimulates release of insulin like growth factor 1 (IGF-1) from the liver, which in turn can stimulate weight gain. IGF-1 remains elevated during administration of the drug. In humans, IGF-1 elevation may act as a negative feedback regulator of GH, but this is unknown in cats. The clinical effects of ELURA in cats are thought to be due to a combination of increased food intake and metabolic changes resulting in weight gain.
Pharmacokinetics
The pharmacokinetic parameters of capromorelin were evaluated in a cross-over study in 4 male and 8 female laboratory cats receiving a single oral dose of ELURA at 2 mg/kg in the fed or fasted state. Following 8 hours of fasting, half the cats were fed a meal of canned food 30 minutes before dosing and the others continued to be fasted until 4 hours post ELURA administration. Blood samples were collected prior to dosing (pre-feeding) and at 0.25, 0.5, 1, 2, 4, 6, 8, 12, and 24 hours post-dosing for determination of serum capromorelin concentrations. Serum concentrations of capromorelin were measured using a liquid chromatography with mass spectrometry detection method. Blood samples were collected prior to dosing (pre-feeding) and at 8, 12, and 24 h post-dosing for determination of serum IGF-1.
Table 2. Mean (Standard Deviation) Pharmacokinetic Parameters for Serum Capromorelin Data were analyzed for only 10 and 6 cats in the fasted and fed groups respectively, because there was an insufficient number of quantifiable serum concentrations for analysis.
aMedian and Range
bInsufficient data to calculate mean and standard deviation for T½
Tmax = time to maximum serum concentration
Cmax =maximum serum concentration
AUClast = area under the curve from the time of dosing to the last quantifiable serum concentration
T½= half-life
Capromorelin was rapidly absorbed following oral administration of ELURA to fasted cats. The Cmax and AUClast for capromorelin were 55% and 43% lower, respectively, in the fed state, as compared to the fasted state. Serum IGF-1 values did not appear to be affected by the feeding state.
Parameter Fasted Fed Tmaxa (hr) 0.25 (0.25-1) (n=10) 0.75 (0.5-4) (n=6) Cmax (ng/mL) 59 ± 42 (n=10) 28 ± 20 (n=6) AUClast (ng*hr/mL) 83 ± 42 (n=10) 51 ± 21 (n=6) T½ (hr) 1.12 ± 0.16 (n=8) NAb -
EFFECTIVENESS:
Effectiveness was demonstrated in a multicenter, prospective, masked, randomized, vehicle-controlled field study. The study enrolled 176 client-owned cats with ≥5% unintended weight loss and a history of chronic kidney disease. The cats enrolled included 96 females and 80 males of various breeds, 4.4 - 22.1 years old with a mean age of 15 years and weighing 1.81 - 6.76 kg. CKD stage was determined based on creatinine at screening according to the International Renal Interest Society (IRIS) 2015 guidelines. All stages were enrolled. Cats were administered ELURA at 2 mg/kg or a matched volume of control once daily by mouth for 56 days. The control was the solution without capromorelin (vehicle control). The primary effectiveness variable was the percent change in body weight from Day 0 to Day 55. Effectiveness was evaluated in 112 cats: 71 cats administered ELURA and 41 cats administered vehicle control. There was a statistically significant difference between the percent change in weight for the ELURA group (+5.2%) compared to the vehicle control group (-1.6%) at Day 55 (p<0.0001). Secondary analysis for percent change in weight at Day 15 and Day 27 demonstrated cats in the ELURA group gained weight throughout the study.
Table 3. Least Squares Mean (Standard Error) Percent Change in Weight from Day 0 a Primary endpoint
Study Day ELURA Vehicle Control Difference (ELURA-Vehicle Control) Day 15 +3.3% (0.4) 0.0% (0.5) +3.3% (0.6) Day 27 +3.8% (0.6) -0.9% (0.7) +4.7% (0.8) Day 55a +5.2% (0.8) -1.6% (1.0) +6.8% (1.2) -
ANIMAL SAFETY:
Margin of Safety Laboratory Study
In a 6-month laboratory study, 32 healthy cats (4 cats/sex/group) approximately 11 months of age were dosed orally once daily in the fasted state with placebo control (0.5 mL/kg water) or ELURA at 2.1 mg/kg (1X), 6.3 mg/kg (3X) or 10.5 mg/kg (5X). Two cats died during the study. One male in the 10.5 mg/kg group died due to urethral obstruction on Day 23; this was unrelated to ELURA administration. One male in the 10.5 mg/kg group developed hyperglycemia and glucosuria on Day 30. This cat was euthanized for clinical decline associated with diabetic ketoacidosis on Day 50. Administration of ELURA resulted in increased body weight (all groups) and increased food consumption (6.3 and 10.5 mg/kg groups). Salivation and intermittent vomiting were observed in placebo and all groups administered ELURA, more frequently in males, and increased in the groups administered ELURA in a dose-dependent manner. The following were observed more frequently in cats in the groups administered ELURA: increased mean corpuscular volume (MCV), increased triglycerides, and soft feces. The following were observed only in cats in the groups administered ELURA: decreased lymphocyte count, decreased hematopoietic cellularity of the bone marrow, focal necrosis of the bone marrow, and mononuclear cell infiltration of the liver. The following changes were observed as trends in groups administered ELURA, although individual values remained within the reference intervals: decreased mean erythrocyte counts, mean hemoglobin concentrations, and mean hematocrits. There were no clinically relevant treatment-related effects on organ weights.
Laboratory Cardiovascular and Blood Glucose Safety Study
A 32-day laboratory study provided information on the cardiovascular and glycemic effects of ELURA in 8 healthy juvenile male cats. Cats had a telemetry device implant for continuous monitoring of cardiovascular variables and blood glucose. Cats were administered vehicle control once daily for 3 days (Days 1-3) followed by ELURA at 2 mg/kg once daily for 28 days (Days 4-31). ELURA administration resulted in transient decreases in heart rate which began after dosing, reached maximal suppression at approximately 1 hour post-dose (lowest individual value was 83 bpm) and returned to baseline within 4 hours. ELURA resulted in transient decreases in direct blood pressure (systolic, diastolic and mean arterial) which began after dosing, reached maximal suppression at approximately 1 hour post-dose (lowest individual value was 72 mmHg systolic) and returned to baseline within 4 hours. The effects on blood pressure were greatest following the first dose of ELURA and decreased in magnitude and frequency, returning to baseline after the ninth dose. The depressive effects of ELURA on heart rate and blood pressure were reversed when the cats were handled by study personnel. ELURA administration resulted in increased blood glucose in 4 cats, with individual variability in magnitude and duration. One cat had a maximum blood glucose of 296 mg/dL recorded 19 hours after the third dose, while values in all other cats remained <160 mg/dL at all times. The effects on glucose resolved after the eighth dose. ELURA administration resulted in increased serum IGF-1, with individual cat variability. Group mean serum IGF-1 was increased on Day 32 compared to the Day -3 baseline. On Day 27, group mean serum IGF-1 was increased 8 hours post-dosing compared to pre-dosing on the same day.
- STORAGE CONDITIONS:
- HOW SUPPLIED:
- Principal Display Panel - 15 mL Carton Label
- Principal Display Panel - 15 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
ELURA
capromorelin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:58198-5545 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Capromorelin (UNII: 0MQ44VUN84) (Capromorelin - UNII:0MQ44VUN84) Capromorelin 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength Methylparaben sodium (UNII: CR6K9C2NHK) Propylparaben sodium (UNII: 625NNB0G9N) Sodium chloride (UNII: 451W47IQ8X) Citric Acid Monohydrate (UNII: 2968PHW8QP) Sucralose (UNII: 96K6UQ3ZD4) Vanillin (UNII: CHI530446X) Povidone K90 (UNII: RDH86HJV5Z) Glycerin (UNII: PDC6A3C0OX) Maltitol (UNII: D65DG142WK) Ammonium Glycyrrhizate (UNII: 3VRD35U26C) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58198-5545-1 1 in 1 CARTON 1 15 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141536 10/16/2020 Labeler - Elanco US Inc. (966985624) Establishment Name Address ID/FEI Business Operations Sterling Wisconsin, LLC 054452136 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Cambrex Charles City, Inc. 782974257 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Argenta Limited 593772127 MANUFACTURE, PACK, LABEL Establishment Name Address ID/FEI Business Operations Halo Pharmaceutical Canada, Inc. 250928632 MANUFACTURE, PACK, LABEL