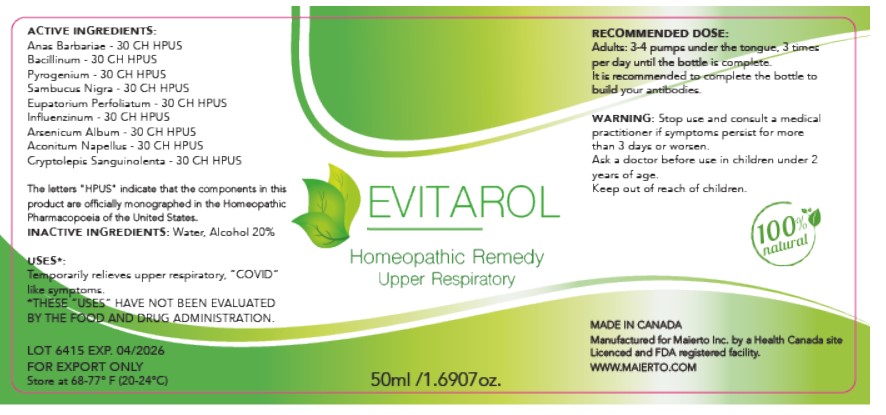
Label: EVITAROL- anas barbariae, bacillinum, pyrogenium, sambucus nigra, eupatorium perfoliatum, influenzinum, arsenicum album, aconitum napellus, cryptolepis sanguinolenta liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 81762-101-50 - Packager: Maierto Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 26, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients
Anas Barbariae 30CH HPUS
Bacillinum 30CH HPUS
Pyrogenium 30CH HPUS
Sambucus Nigra 30CH HPUS
Eupatorium Perfoliatum 30CH HPUS
Influenzinum 30CH
Arsenicum Album 30CH HPUS
Aconitum Napellus 30CH HPUS
Cryptolepis Sanguinolenta 30CH HPUS
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
-
PURPOSE
Anas Barbariae 30CH HPUS ..... reduces the duration of and severity of flu-like symptoms
Bacillinum 30CH HPUS ….. helps in treatment of tuberculosis and relief from other respiratory disorders like asthma and bronchitis
Pyrogenium 30CH HPUS ….. helps promote and maintain a healthy upper respiratory tract, and temporarily alleviates symptoms associated with general nasal and sinus congestion.
Sambucus Nigra 30CH HPUS ….. boosts immune system, and helps prevent and ease cold and flu-like symptoms
Eupatorium Perfoliatum 30CH HPUS ….. helps to reduce fever, and relieves stiffness and bone pain
Influenzinum 30CH HPUS …. prevention and relief of flu-like symptoms
Arsenicum Album 30CH HPUS ….. reduces inflammation in body
Aconitum Napellus 30CH HPUS ….. alleviates fever and chills
Cryptolepis Sanguinolenta 30CH HPUS ….. antibacterial and anti-inflammatory activity
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EVITAROL
anas barbariae, bacillinum, pyrogenium, sambucus nigra, eupatorium perfoliatum, influenzinum, arsenicum album, aconitum napellus, cryptolepis sanguinolenta liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81762-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BOVINE TUBERCULIN (UNII: HKD62G79N5) (BOVINE TUBERCULIN - UNII:HKD62G79N5) BOVINE TUBERCULIN 30 [hp_C] in 30 [hp_C] ACONITUM NAPELLUS WHOLE (UNII: U0NQ8555JD) (ACONITUM NAPELLUS WHOLE - UNII:U0NQ8555JD) ACONITUM NAPELLUS WHOLE 30 [hp_C] in 30 [hp_C] CRYPTOLEPIS SANGUINOLENTA WHOLE (UNII: 9Q3JWJ3GXG) (CRYPTOLEPIS SANGUINOLENTA WHOLE - UNII:9Q3JWJ3GXG) CRYPTOLEPIS SANGUINOLENTA WHOLE 30 [hp_C] in 30 [hp_C] CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE (UNII: RN2HC612GY) (CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE - UNII:RN2HC612GY) CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE 30 [hp_C] in 30 [hp_C] INFLUENZA A VIRUS A/HONG KONG/2671/2019 (H3N2) NIB-121 NEURAMINIDASE ANTIGEN (UV, FORMALDEHYDE INACTIVATED) (UNII: 2828WNW8HO) (INFLUENZA A VIRUS A/HONG KONG/2671/2019 (H3N2) NIB-121 NEURAMINIDASE ANTIGEN (UV, FORMALDEHYDE INACTIVATED) - UNII:2828WNW8HO) INFLUENZA A VIRUS A/HONG KONG/2671/2019 (H3N2) NIB-121 NEURAMINIDASE ANTIGEN (UV, FORMALDEHYDE INACTIVATED) 30 [hp_C] in 30 [hp_C] INFLUENZA B VIRUS B/WASHINGTON/02/2019 ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: VEH9U90EHX) (INFLUENZA B VIRUS B/WASHINGTON/02/2019 ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:VEH9U90EHX) INFLUENZA B VIRUS B/WASHINGTON/02/2019 ANTIGEN (FORMALDEHYDE INACTIVATED) 30 [hp_C] in 30 [hp_C] SAMBUCUS NIGRA FLOWERING TOP (UNII: CT03BSA18U) (SAMBUCUS NIGRA FLOWERING TOP - UNII:CT03BSA18U) SAMBUCUS NIGRA FLOWERING TOP 30 [hp_C] in 30 [hp_C] ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 30 [hp_C] in 30 [hp_C] INFLUENZA A VIRUS A/GUANGDONG-MAONAN/SWL1536/2019 CNIC-1909 (H1N1) ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: NY1FF92M1E) (INFLUENZA A VIRUS A/GUANGDONG-MAONAN/SWL1536/2019 CNIC-1909 (H1N1) ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:NY1FF92M1E) INFLUENZA A VIRUS A/GUANGDONG-MAONAN/SWL1536/2019 CNIC-1909 (H1N1) ANTIGEN (FORMALDEHYDE INACTIVATED) 30 [hp_C] in 30 [hp_C] RANCID BEEF (UNII: 29SUH5R3HU) (RANCID BEEF - UNII:29SUH5R3HU) RANCID BEEF 30 [hp_C] in 30 [hp_C] INFLUENZA B VIRUS B/PHUKET/3073/2013 ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: B93BQX9789) (INFLUENZA B VIRUS B/PHUKET/3073/2013 ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:B93BQX9789) INFLUENZA B VIRUS B/PHUKET/3073/2013 ANTIGEN (FORMALDEHYDE INACTIVATED) 30 [hp_C] in 30 [hp_C] EUPATORIUM PERFOLIATUM FLOWERING TOP (UNII: 1W0775VX6E) (EUPATORIUM PERFOLIATUM FLOWERING TOP - UNII:1W0775VX6E) EUPATORIUM PERFOLIATUM FLOWERING TOP 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81762-101-50 1 in 1 BOX 04/26/2021 1 50 [hp_C] in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/26/2021 Labeler - Maierto Inc (204274278)

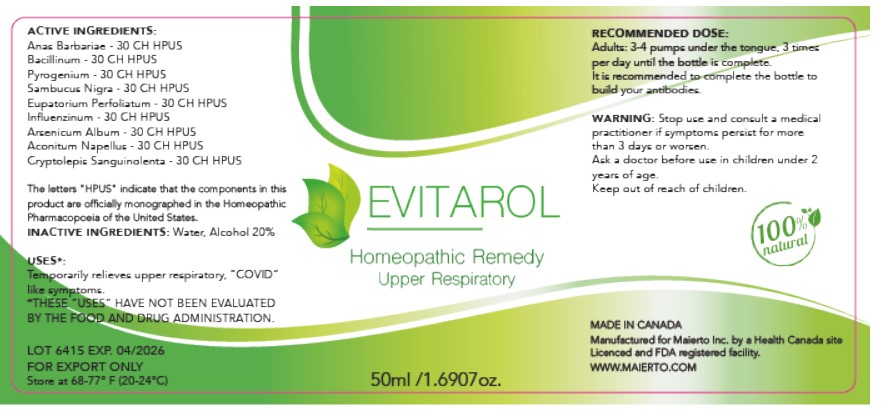
 Evitarol
Evitarol