Label: NATURAL HYDRATING SHIELD SPF 30- zinc oxide cream
- NDC Code(s): 79228-0000-0
- Packager: Group Fourteen Operations Pty Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
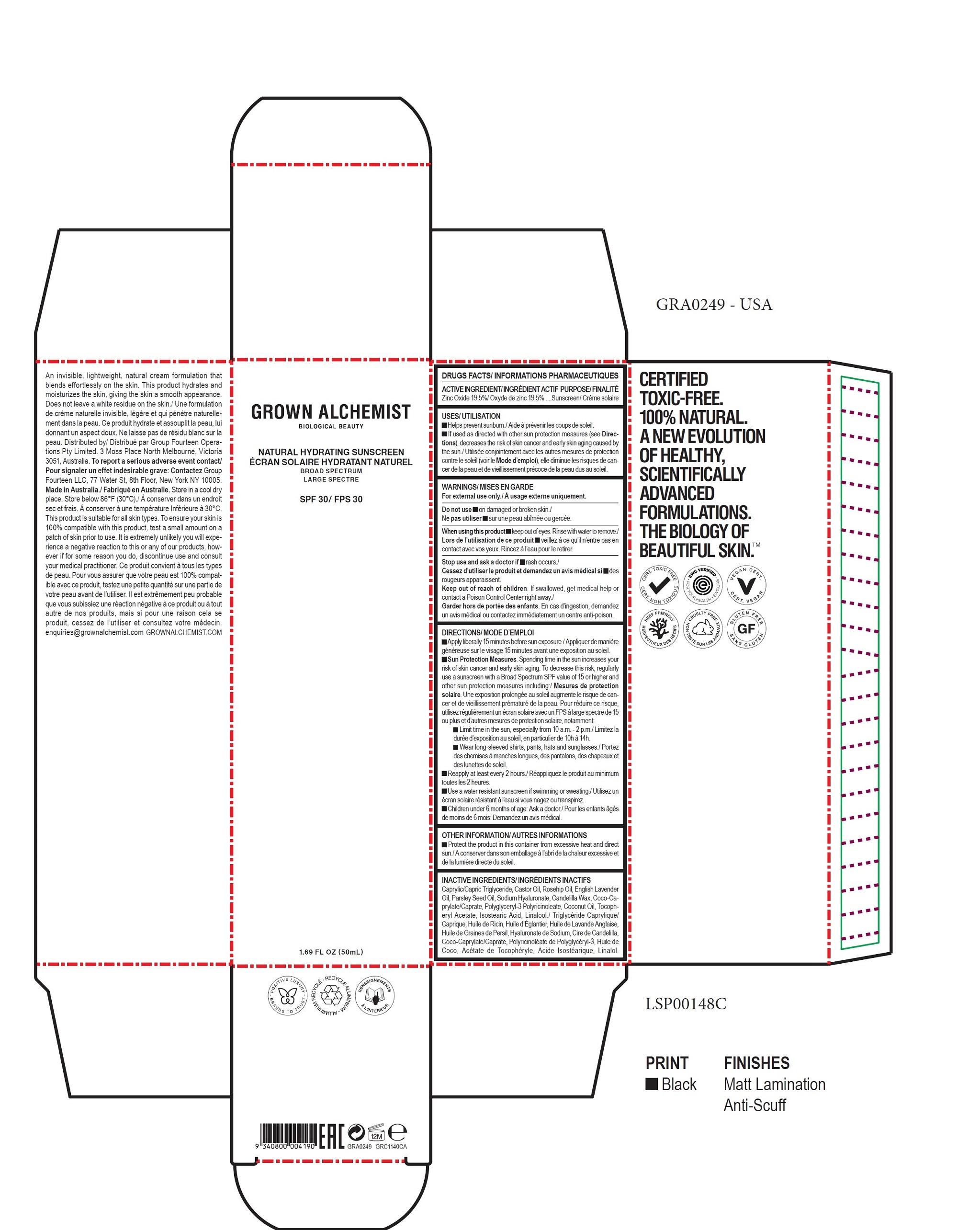
- DRUGS FACTS
- ACTIVE INGREDIENT
- USES
- WARNINGS
-
DIRECTIONS
- Apply liberally 15 minutes before sun exposure.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly
use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: - Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Children under 6 months of age: Ask a doctor.
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
NATURAL HYDRATING SHIELD SPF 30
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79228-0000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 195 mg in 1 mL Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CASTOR OIL (UNII: D5340Y2I9G) ROSA CANINA FRUIT OIL (UNII: CR7307M3QZ) ENGLISH LAVENDER OIL (UNII: ZBP1YXW0H8) PARSLEY SEED OIL (UNII: XB8Q500O0Z) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CANDELILLA WAX (UNII: WL0328HX19) COCOYL CAPRYLOCAPRATE (UNII: 8D9H4QU99H) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ISOSTEARIC ACID (UNII: X33R8U0062) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79228-0000-0 50 mL in 1 TUBE; Type 0: Not a Combination Product 01/12/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/12/2020 Labeler - Group Fourteen Operations Pty Limited (757031294)