Label: RECARBRIO- imipenem anhydrous, cilastatin, and relebactam anhydrous injection, powder, for solution
- NDC Code(s): 0006-3856-01, 0006-3856-02
- Packager: Merck Sharp & Dohme LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated July 26, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RECARBRIO safely and effectively. See full prescribing information for RECARBRIO.
RECARBRIO™ (imipenem, cilastatin, and relebactam) for injection, for intravenous use
Initial U.S. Approval: 2019INDICATIONS AND USAGE
RECARBRIO is a combination of imipenem, a penem antibacterial, cilastatin, a renal dehydropeptidase inhibitor, and relebactam, a beta-lactamase inhibitor, indicated in patients 18 years of age and older for the treatment of the following infections caused by susceptible gram-negative microorganisms:
- Hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP). (1.1)
- Complicated urinary tract infections, including pyelonephritis (cUTI) in patients who have limited or no alternative treatment options. (1.2)
- Complicated intra-abdominal infections (cIAI) in patients who have limited or no alternative treatment options. (1.3)
Approval of the cUTI and cIAI indications is based on limited clinical safety and efficacy data for RECARBRIO. (1.2, 1.3, 14)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of RECARBRIO and other antibacterial drugs, RECARBRIO should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1.3)
DOSAGE AND ADMINISTRATION
- Administer RECARBRIO 1.25 grams (imipenem 500 mg, cilastatin 500 mg, relebactam 250 mg) by intravenous (IV) infusion over 30 minutes every 6 hours in patients 18 years of age and older with creatinine clearance (CLcr) 90 mL/min or greater. (2.1)
- Dosage adjustment in patients with renal impairment. (2.2)
Estimated Creatinine Clearance (mL/min)* Recommended Dose of RECARBRIO (imipenem/cilastatin/relebactam) (mg) administered by IV infusion over 30 minutes every 6 hours - *
- CLcr calculated using the Cockcroft-Gault formula.
60 to 89 1 gram (imipenem 400 mg, cilastatin 400 mg, and relebactam 200 mg 30 to 59 0.75 grams (imipenem 300 mg, cilastatin 300 mg, and relebactam 150 mg 15 to 29 0.5 grams (imipenem 200 mg, cilastatin 200 mg, and relebactam 100 mg End Stage Renal Disease on Hemodialysis 0.5 grams (imipenem 200 mg, cilastatin 200 mg, and relebactam 100 mg - Patients with CLcr less than 15 mL/min should not receive RECARBRIO unless hemodialysis is instituted within 48 hours. (2.2)
- See Full Prescribing Information for instructions for constituting supplied dry powder and subsequent required dilution. (2.3, 2.4)
- See Full Prescribing Information for drug compatibilities and incompatibilities. (2.6, 2.7)
DOSAGE FORMS AND STRENGTHS
RECARBRIO 1.25 grams for injection is supplied as sterile powder for constitution in a single-dose vial containing imipenem 500 mg (anhydrate equivalent), cilastatin 500 mg (free acid equivalent), and relebactam 250 mg (anhydrate equivalent). (3)
CONTRAINDICATIONS
RECARBRIO is contraindicated in patients with a history of known severe hypersensitivity to any component of RECARBRIO. (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: Hypersensitivity reactions have been reported in patients receiving beta lactam drugs. Discontinue RECARBRIO immediately if a hypersensitivity reaction occurs. (5.1)
- Seizures and Central Nervous System Adverse Reactions: CNS adverse reactions such as seizures have been reported with imipenem/cilastatin, a component of RECARBRIO. If focal tremors, myoclonus, or seizures occur, evaluate patients, to determine whether RECARBRIO should be discontinued. (5.2)
- Increased Seizure Potential Due to Interaction with Valproic Acid: Concomitant use of RECARBRIO with valproic acid or divalproex sodium may reduce the serum concentration of valproic acid which may increase the risk of breakthrough seizures. Avoid concomitant use or consider alternative antibacterial drugs other than carbapenems. (5.3, 7.2)
- Clostridioides difficile-Associated Diarrhea (CDAD): Has been reported with RECARBRIO. Evaluate if diarrhea occurs. (5.4)
ADVERSE REACTIONS
- HABP/VABP Patients: The most frequently reported adverse reactions occurring in greater than or equal to 5% of patients treated with RECARBRIO were alanine aminotransferase increased, aspartate aminotransferase increased, anemia, diarrhea, hypokalemia, and hyponatremia. (6)
- cUTI and cIAI Patients: The most frequently reported adverse reactions occurring in greater than or equal to 2% of patients treated with imipenem/cilastatin plus relebactam 250 mg, the components of RECARBRIO, were diarrhea, nausea, headache, vomiting, alanine aminotransferase increased, aspartate aminotransferase increased, phlebitis/infusion site reactions, pyrexia, and hypertension. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP)
1.2 Complicated Urinary Tract Infections (cUTI), including Pyelonephritis
1.3 Complicated Intra-abdominal Infections (cIAI)
1.4 Usage
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adults
2.2 Dosage Adjustments in Patients with Renal Impairment
2.3 Preparation of RECARBRIO Solution for Intravenous Administration
2.4 Preparation of RECARBRIO Solution for Intravenous Administration in Patients with Renal Impairment
2.5 Storage of Constituted Solution
2.6 Compatible Injectable Drug Products
2.7 Incompatible Injectable Drug Products
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Seizures and Other Central Nervous System (CNS) Adverse Reactions
5.3 Increased Seizure Potential Due to Interaction with Valproic Acid
5.4 Clostridioides difficile-Associated Diarrhea (CDAD)
5.5 Development of Drug-resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Ganciclovir
7.2 Valproic Acid
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia
14.2 Complicated Urinary Tract Infections, including Pyelonephritis and Complicated Intra-abdominal Infections
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP)
RECARBRIO™ is indicated for the treatment of patients 18 years of age and older with hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia, caused by the following susceptible gram-negative microorganisms: Acinetobacter calcoaceticus-baumannii complex, Enterobacter cloacae, Escherichia coli, Haemophilus influenzae, Klebsiella aerogenes, Klebsiella oxytoca, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Serratia marcescens.
1.2 Complicated Urinary Tract Infections (cUTI), including Pyelonephritis
RECARBRIO is indicated in patients 18 years of age and older who have limited or no alternative treatment options, for the treatment of complicated urinary tract infections (cUTI), including pyelonephritis, caused by the following susceptible gram-negative microorganisms: Enterobacter cloacae, Escherichia coli, Klebsiella aerogenes, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
Approval of this indication is based on limited clinical safety and efficacy data for RECARBRIO [see Clinical Studies (14.2)].
1.3 Complicated Intra-abdominal Infections (cIAI)
RECARBRIO is indicated in patients 18 years of age and older who have limited or no alternative treatment options for the treatment of complicated intra-abdominal infections (cIAI) caused by the following susceptible gram-negative microorganisms: Bacteroides caccae, Bacteroides fragilis, Bacteroides ovatus, Bacteroides stercoris, Bacteroides thetaiotaomicron, Bacteroides uniformis, Bacteroides vulgatus, Citrobacter freundii, Enterobacter cloacae, Escherichia coli, Fusobacterium nucleatum, Klebsiella aerogenes, Klebsiella oxytoca, Klebsiella pneumoniae, Parabacteroides distasonis, and Pseudomonas aeruginosa.
Approval of this indication is based on limited clinical safety and efficacy data for RECARBRIO [see Clinical Studies (14.2)].
1.4 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of RECARBRIO and other antibacterial drugs, RECARBRIO should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adults
The recommended dosage of RECARBRIO is 1.25 grams (imipenem 500 mg, cilastatin 500 mg, and relebactam 250 mg) administered by intravenous (IV) infusion over 30 minutes every 6 hours in patients 18 years of age and older with creatinine clearance (CLcr) of 90 mL/min or greater. A dose reduction is recommended for patients with CLcr less than 90 mL/min (Table 1) [see Dosage and Administration (2.2)]. The severity and location of infection, as well as clinical response should guide the duration of therapy. The recommended duration of treatment with RECARBRIO is 4 days to 14 days.
2.2 Dosage Adjustments in Patients with Renal Impairment
Dosage adjustment is recommended in patients with renal impairment. Patients who have a CLcr less than 90 mL/min require dosage reduction of RECARBRIO (Table 1). For patients with fluctuating renal function, CLcr should be monitored.
Table 1: Dosage of RECARBRIO for Adult Patients with Renal Impairment Estimated CLcr (mL/min)* Recommended Dosage of RECARBRIO (imipenem/cilastatin and relebactam) (mg)† Dosing Interval RECARBRIO is provided as a single vial in a fixed-dose combination; the dose for each component will be adjusted equally during preparation [see Dosage and Administration (2.4)]. 60 to 89 1 gram (imipenem 400 mg, cilastatin 400 mg, and relebactam 200 mg) Every 6 hours 30 to 59 0.75 grams (imipenem 300 mg, cilastatin 300 mg, and relebactam 150 mg) Every 6 hours 15 to 29 0.5 grams (imipenem 200 mg, cilastatin 200 mg, and relebactam 100 mg) Every 6 hours End Stage Renal Disease (ESRD) on Hemodialysis‡ 0.5 grams (imipenem 200 mg, cilastatin 200 mg, and relebactam 100 mg) Every 6 hours Patients with CLcr less than 15 mL/min should not receive RECARBRIO unless hemodialysis is instituted within 48 hours. There is inadequate information to recommend usage of RECARBRIO for patients undergoing peritoneal dialysis.
Imipenem, cilastatin, and relebactam are cleared from the circulation during hemodialysis. For patients maintained on hemodialysis, administer RECARBRIO after hemodialysis and at intervals timed from the end of that hemodialysis session.
2.3 Preparation of RECARBRIO Solution for Intravenous Administration
RECARBRIO is supplied as a dry powder in a single-dose vial that must be constituted and further diluted using aseptic technique prior to intravenous infusion. To prepare the infusion solution, contents of the vial must be constituted with the appropriate diluent as instructed below. A list of appropriate diluents is as follows:
- 0.9% Sodium Chloride Injection, USP
- 5% Dextrose Injection, USP
- 5% Dextrose Injection, USP + 0.9% Sodium Chloride Injection, USP
- 5% Dextrose Injection, USP + 0.45% Sodium Chloride Injection, USP
- 5% Dextrose Injection, USP + 0.225% Sodium Chloride Injection, USP
RECARBRIO has low aqueous solubility. To ensure complete dissolution of RECARBRIO it is important to adhere to the following instructions:
- Step 1) For diluents available in 100 mL prefilled infusion bags, proceed to step 2. For diluents not available in 100 mL prefilled infusion bags, aseptically withdraw 100 mL of the desired diluent and transfer it to an empty infusion bag, then proceed to step 2.
- Step 2) Withdraw 20 mL (as two 10 mL aliquots) of diluent from the appropriate infusion bag and constitute the vial with one 10 mL aliquot of the diluent. The constituted suspension is for intravenous infusion only after dilution in an appropriate infusion solution.
- Step 3) After constitution, shake vial well and transfer resulting suspension into the remaining 80 mL of the infusion bag.
- Step 4) Add the second 10 mL aliquot of infusion diluent to the vial and shake well to ensure complete transfer of vial contents; repeat transfer of the resulting suspension to the infusion solution before administering. Agitate the resulting mixture until clear.
Constituted solutions of RECARBRIO range from colorless to yellow. Variations of color within this range do not affect the potency of the product.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard if discoloration or visible particles are observed.
The above instructions for preparation of RECARBRIO solution for intravenous administration must be followed for all patients, irrespective of the intended patient's renal function. The volume of this prepared RECARBRIO solution to be administered to patients is determined based on renal function [see Dosage and Administration (2.4)].
2.4 Preparation of RECARBRIO Solution for Intravenous Administration in Patients with Renal Impairment
For patients with renal impairment, prepare a reduced dose of RECARBRIO (1 gram, 0.75 grams, or 0.5 grams) [see Dosage and Administration (2.2)] by preparing a 100 mL solution containing 1.25 grams (as described above in Section 2.3) then withdrawing and discarding the excess according to Table 2.
Table 2: Preparation of Reduced RECARBRIO Doses for Intravenous Administration in Patients with Renal Impairment Creatinine Clearance (mL/min) Dosage of RECARBRIO (imipenem/cilastatin/relebactam) After preparation as instructed above, remove from the 100 mL prepared bag the volume indicated below and discard Resulting volume that provides the indicated reduced dose 60 to 89 1 gram (imipenem 400 mg, cilastatin 400 mg, and relebactam 200 mg) 20 mL 80 mL 30 to 59 0.75 grams (imipenem 300 mg, cilastatin 300 mg, and relebactam 150 mg) 40 mL 60 mL 15 to 29 or ESRD on hemodialysis 0.5 grams (imipenem 200 mg, cilastatin 200 mg, and relebactam 100 mg) 60 mL 40 mL 2.5 Storage of Constituted Solution
RECARBRIO, as supplied in single-dose glass vials upon constitution with the appropriate diluent and following further dilution in the infusion bag, maintains satisfactory potency for at least 2 hours at room temperature (up to 30°C) or for at least 24 hours under refrigeration at 2°C to 8°C (36°F to 46°F). Do not freeze solutions of RECARBRIO.
2.6 Compatible Injectable Drug Products
Compatible Drug Products
The physical compatibility of RECARBRIO with selected injectable drug products was evaluated in two commonly available diluents. Compatible drugs with the corresponding compatible diluent (i.e., 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP) are listed below. RECARBRIO should not be co-administered through the same intravenous line (or cannula), with other drug products not listed below, as no compatibility data are available. Refer to the respective prescribing information of the co-administered drug(s) to confirm compatibility of simultaneous co-administration.
List of Compatible Injectable Drugs for use with 5% Dextrose USP or 0.9% Sodium Chloride USP Injection as Diluents
- dexmedetomidine
- dopamine
- epinephrine
- fentanyl
- heparin
- midazolam
- norepinephrine
- phenylephrine
-
3 DOSAGE FORMS AND STRENGTHS
RECARBRIO (imipenem, cilastatin, and relebactam) for injection, 1.25 grams is supplied as a white to light yellow sterile powder for constitution in a single-dose glass vial containing imipenem 500 mg (equivalent to 530 mg imipenem monohydrate), cilastatin 500 mg (equivalent to 531 mg cilastatin sodium), and relebactam 250 mg (equivalent to 263 mg relebactam monohydrate).
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving therapy with beta lactams. Before initiating therapy with RECARBRIO, careful inquiry should be made concerning previous hypersensitivity reactions to carbapenems, penicillins, cephalosporins, other beta lactams, and other allergens. If a hypersensitivity reaction to RECARBRIO occurs, discontinue the therapy immediately.
RECARBRIO is contraindicated in patients with a history of severe hypersensitivity to any component of RECARBRIO [see Contraindications (4)].
5.2 Seizures and Other Central Nervous System (CNS) Adverse Reactions
CNS adverse reactions, such as seizures, confusional states, and myoclonic activity, have been reported during treatment with imipenem/cilastatin, a component of RECARBRIO, especially when recommended dosages of imipenem were exceeded. These have been reported most commonly in patients with CNS disorders (e.g., brain lesions or history of seizures) and/or compromised renal function.
Anticonvulsant therapy should be continued in patients with known seizure disorders. If CNS adverse reactions including seizures occur, patients should undergo a neurological evaluation to determine whether RECARBRIO should be discontinued.
5.3 Increased Seizure Potential Due to Interaction with Valproic Acid
Concomitant use of RECARBRIO, with valproic acid or divalproex sodium may increase the risk of breakthrough seizures. Avoid concomitant use of RECARBRIO with valproic acid or divalproex sodium or consider alternative antibacterial drugs other than carbapenems [see Drug Interactions (7.2)].
5.4 Clostridioides difficile-Associated Diarrhea (CDAD)
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including RECARBRIO, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial drug treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described in greater detail in the Warnings and Precautions section.
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Seizures and Other Central Nervous System Adverse Reactions [see Warnings and Precautions (5.2)]
- Increased Seizure Potential Due to Interaction with Valproic Acid [see Warnings and Precautions (5.3)]
- Clostridioides difficile-Associated Diarrhea (CDAD) [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Overview of the Safety Evaluation of RECARBRIO
Safety was primarily evaluated in three active-controlled, double-blind trials in HABP/VABP, cUTI, and cIAI (Trials 1, 2, and 3, respectively).
In the HABP/VABP trial (Trial 1), patients were treated with either RECARBRIO or piperacillin and tazobactam (4.5 grams).
In the cUTI trial (Trial 2) and cIAI trial (Trial 3), patients in the treatment arms were treated with either imipenem 500 mg/cilastatin 500 mg and relebactam 250 mg or imipenem 500 mg/cilastatin 500 mg and relebactam 125 mg (not an approved dose), and patients in the control arm were treated with imipenem 500 mg/cilastatin 500 mg plus placebo (IV normal saline). Across Trials 2 and 3, the mean duration of IV therapy in patients treated with imipenem/cilastatin plus relebactam 250 mg was approximately 7 days.
Clinical Trial Experience in Patients with HABP/VABP
Trial 1 included 266 adult patients treated with RECARBRIO and 269 patients treated with piperacillin and tazobactam (4.5 grams) administered intravenously over 30 minutes every 6 hours. The mean age was 60 years, 43% of patients were 65 years of age and older, 31% were female and 22% had polymicrobial infection. The mean Acute Physiology and Chronic Health Evaluation (APACHE) II score was 15 and 48% of patients had an APACHE II score greater than or equal to 15 at baseline. Overall, 260 (49%) patients were ventilated at enrollment, including 194 (36%) patients with VABP and 66 (12%) patients with ventilated HABP.
Clinical Trial Experience in Patients with cUTI including, Pyelonephritis
Trial 2 included 198 adult patients treated with imipenem/cilastatin and relebactam (99 patients each with imipenem 500 mg/cilastatin 500 mg plus relebactam 125 mg or relebactam 250 mg) and 100 patients treated with imipenem 500 mg/cilastatin 500 mg, administered intravenously over 30 minutes every 6 hours. After a minimum of 4 days of IV therapy, patients could be switched to oral ciprofloxacin (500 mg daily every 12 hours) to complete the treatment course of 4 to 14 days total (IV plus oral), at the discretion of the investigator. The mean age was 56 years, 40% of patients were 65 years of age and older, 16% were 75 years of age and older, 50% were female, and approximately 18% had moderate to severe renal impairment.
Clinical Trial Experience in Patients with cIAI
Trial 3 included 233 adult patients treated with imipenem/cilastatin plus relebactam (116 subjects with imipenem 500 mg/cilastatin 500 mg and relebactam 125 mg and 117 subjects with imipenem 500 mg/cilastatin 500 mg plus relebactam 250 mg), and 114 patients treated with imipenem 500 mg/cilastatin 500 mg, administered intravenously over 30 minutes every 6 hours for 4 to 14 days, at the discretion of the investigator. The mean age was 49 years, 23% of the patients were 65 years of age and older, 9.8% were 75 years of age and older, and 42% were female.
Serious Adverse Reactions and Adverse Reactions Leading to Discontinuation
In Trial 1, serious adverse reactions occurred in 27% (71/266) of patients receiving RECARBRIO and 32% (86/269) of patients receiving piperacillin and tazobactam. Adverse reactions leading to death were reported in 15% (40/266) of patients receiving RECARBRIO and 21% (57/269) of patients receiving piperacillin and tazobactam.
Adverse reactions leading to discontinuation occurred in 5.6% (15/266) of patients receiving imipenem 500 mg/cilastatin 500 mg/relebactam 250 mg and 8.2% (22/269) of patients receiving piperacillin and tazobactam.
In Trials 2 and 3, serious adverse reactions occurred in 3.2% (7/216) of patients receiving imipenem 500 mg/cilastatin 500 mg plus relebactam 250 mg and 5.1% (11/214) of patients receiving imipenem 500 mg/cilastatin 500 mg. There were no deaths reported in patients receiving imipenem 500 mg/cilastatin 500 mg plus relebactam 250 mg or imipenem 500 mg/cilastatin 500 mg alone. Deaths were reported in 1.4% (3/215) of patients receiving imipenem 500 mg/cilastatin 500 mg plus relebactam 125 mg (not an approved dose).
Adverse reactions leading to discontinuation occurred in 1.9% (4/216) of patients receiving imipenem 500 mg/cilastatin 500 mg plus relebactam 250 mg and 2.3% (5/214) of patients receiving imipenem 500 mg/cilastatin 500 mg.
Common Adverse Reactions
In Trial 1, adverse reactions occurred during the protocol-specified follow-up period, which was IV therapy plus 14 days following completion of therapy, in 85% (226/266) of patients receiving RECARBRIO and 87% (233/269) of patients receiving piperacillin and tazobactam. Table 3 lists the most common adverse reactions occurring in ≥4% of patients receiving imipenem 500 mg/cilastatin 500 mg/relebactam 250 mg or piperacillin and tazobactam in Trial 1.
Table 3: Adverse Reactions Occurring in Greater Than or Equal to 4% of HABP/VABP Patients Receiving RECARBRIO in Trial 1 Adverse Reaction RECARBRIO*
(N=266)
N (%)Piperacillin/Tazobactam†
(N=269)
N (%)- *
- RECARBRIO, IV every 6 hours.
- †
- Piperacillin 4000 mg and Tazobactam 500 mg (4.5 grams), IV every 6 hours.
- ‡
- Hypokalemia includes hypokalemia and blood potassium decreased.
- §
- Hyponatremia includes hyponatremia and blood sodium decreased.
- ¶
- Rash includes rash, rash erythematous, and rash generalized.
Blood and lymphatic system disorders Anemia 28 (10.5%) 27 (10.0%) Gastrointestinal disorders Constipation 11 (4.1%) 3 (1.1%) Diarrhea 21 (7.9%) 30 (11.2%) General disorders and administration site conditions Pyrexia 11 (4.1%) 20 (7.4%) Laboratory investigations Alanine aminotransferase increased 26 (9.8%) 19 (7.1%) Aspartate aminotransferase increased 31 (11.7%) 20 (7.4%) Metabolism and nutrition disorders Hypokalemia‡ 21 (7.9%) 26 (9.7%) Hyponatremia§ 17 (6.4%) 3 (1.1%) Skin and subcutaneous tissue disorders Rash¶ 11 (4.1%) 5 (1.9%) Less Common Adverse Reactions Reported in Trial 1
The following selected adverse reaction was reported in RECARBRIO-treated subjects at a rate of less than 4%:
Blood and lymphatic system disorders: thrombocytopenia
In Trials 2 and 3, adverse reactions occurred during the protocol-specified follow-up period, which was IV therapy plus 14 days following completion of therapy, in 39% (85/216) of patients receiving imipenem 500 mg/cilastatin 500 mg plus relebactam 250 mg and 36% (77/214) of patients receiving imipenem 500 mg/cilastatin 500 mg. Table 4 lists the most common adverse reactions occurring in ≥1% of patients receiving imipenem 500 mg/cilastatin 500 mg plus relebactam 250 mg or imipenem 500 mg/cilastatin 500 mg in Trials 2 and 3.
Table 4: Adverse Reactions Occurring in Greater Than or Equal to 1% of cUTI and cIAI Patients Receiving Imipenem/Cilastatin plus Relebactam 250 mg or Imipenem/Cilastatin in Trials 2 and 3 Adverse Reaction Imipenem/Cilastatin and Relebactam 250 mg*
(N=216)
N (%)IMI + Placebo†
(N=214)
N (%)- *
- Imipenem/Cilastatin (500 mg/500 mg) + Relebactam (250 mg), IV every 6 hours.
- †
- Imipenem/Cilastatin (500 mg/500 mg) + Placebo, IV every 6 hours.
- ‡
- Anemia includes anemia and hemoglobin decreased.
- §
- Infusion site reactions include infusion site phlebitis, infusion site erythema, and infusion site pain.
- ¶
- Central nervous system adverse reactions include agitation, apathy, confusional states, delirium, disorientation, slow speech, and somnolence.
- #
- Hypertension includes hypertension and blood pressure increased.
Blood and lymphatic system disorders Anemia‡ 2 (1%) 4 (2%) Gastrointestinal disorders Diarrhea 12 (6%) 9 (4%) Nausea 12 (6%) 12 (6%) Vomiting 7 (3%) 4 (2%) General disorders and administration site conditions Phlebitis/Infusion site reactions§ 5 (2%) 3 (1%) Pyrexia 5 (2%) 3 (1%) Laboratory Investigations Alanine aminotransferase increased 7 (3%) 4 (2%) Aspartate aminotransferase increased 6 (3%) 3 (1%) Lipase increased 3 (1%) 4 (2%) Blood creatinine increased 1 (<1%) 3 (1%) Nervous system disorders Headache 9 (4%) 5 (2%) Central nervous system adverse reactions¶ 2 (1%) 5 (2%) Vascular disorders Hypertension# 4 (2%) 6 (3%) Other Adverse Reactions Associated with Imipenem/Cilastatin
Adverse reactions reported with imipenem/cilastatin, a component of RECARBRIO, in clinical studies or during post-marketing experience are listed below. These adverse reactions are not listed above for patients treated with RECARBRIO in Trial 1 or imipenem 500 mg/cilastatin 500 mg plus relebactam 250 mg in Trials 2 and 3.
Blood and Lymphatic System Disorders: agranulocytosis, increased eosinophils, hemolytic anemia
Nervous System Disorders: seizure
Hepatobiliary Disorders: hepatic failure, jaundice
Laboratory Investigations: blood lactate dehydrogenase increased, coombs test positive, eosinophil count increased.
-
7 DRUG INTERACTIONS
7.1 Ganciclovir
Generalized seizures have been reported in patients who received ganciclovir concomitantly with imipenem/cilastatin, a component of RECARBRIO. Ganciclovir should not be used concomitantly with RECARBRIO unless the potential benefits outweigh the risks.
7.2 Valproic Acid
Based on case reports in the literature concomitant use of carbapenems, including imipenem/cilastatin, components of RECARBRIO, with valproic acid or divalproex sodium may decrease valproic acid concentrations which may increase the risk of breakthrough seizures [see Warnings and Precautions (5.3)]. Although the mechanism of this interaction is unknown, data from in vitro and animal studies suggest that carbapenems may inhibit the hydrolysis of valproic acid's glucuronide metabolite (VPA-g) back to valproic acid, thus decreasing the serum concentrations of valproic acid. Avoid concomitant use of RECARBRIO with valproic acid or divalproex sodium. Consider alternative antibacterials other than carbapenems to treat infections in patients whose seizures are well controlled on valproic acid or divalproex sodium.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Embryonic loss was observed in monkeys treated with imipenem/cilastatin, and fetal abnormalities were observed in relebactam-treated mice; therefore, advise pregnant women of the potential risks to pregnancy and the fetus. There are insufficient human data to establish whether there is a drug-associated risk for major birth defects, miscarriage, or adverse maternal or fetal outcomes with RECARBRIO, imipenem, cilastatin, or relebactam in pregnant women.
Developmental toxicity studies with imipenem and cilastatin (alone or in combination) administered parenterally during organogenesis to mice, rats, rabbits, and monkeys at doses 1 to 5 times the maximum recommended human dose (MRHD of imipenem 500 mg/cilastatin 500 mg every 6 hours for total daily doses of imipenem 2000 mg/cilastatin 2000 mg) based on body surface area comparison, showed no drug-induced fetal malformations. Embryofetal development studies with imipenem/cilastatin administered to cynomolgus monkeys at doses similar to the MRHD (based on body surface area comparison) showed an increase in embryonic loss. In an embryofetal study, parental administration of relebactam to pregnant mice during the period of organogenesis was associated with a non-dose responsive increase in the litter incidence of cleft palate at a plasma relebactam exposure approximately equal to the human exposure at the MRHD (250 mg every 6 hours for a daily dose of 1000 mg) and an increased percent litter incidence of total skeletal malformations at a plasma exposure approximately 6 times the human exposure at the MRHD. Reproductive studies with relebactam administered parenterally to pregnant rats and rabbits during the period of organogenesis at plasma exposures up to 7 and 24 times, respectively, the plasma exposure in humans at the MRHD showed no adverse effects on pregnancy or embryofetal development. Relebactam administered to rats during gestation through lactation was not associated with fetal toxicity, developmental delays, or impaired reproduction in first generation offspring at plasma exposures equivalent to 8 times the human exposure at the MRHD (see Data).
The background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The estimated background risk of major birth defects is 2 to 4% and miscarriage is 15 to 20% of clinically recognized pregnancies within the U.S. general population.
Data
Animal Data
Imipenem and Cilastatin
Reproductive toxicity studies with imipenem and cilastatin (alone or in combination) administered parenterally to mice, rats, and rabbits showed no evidence of effects on embryofetal (mice, rats, and rabbits) or pre/postnatal (rats) development. In embryofetal development studies, imipenem was administered intravenously to rats (gestation days (GD) 7 to 17), and rabbits (GD 6 to 18), at doses up to 900 and 60 mg/kg/day, respectively, approximately 4 and 0.6 times the MRHD (based on body surface area comparison). Cilastatin was administered subcutaneously to rats (GD 6 to 17) and intravenously to rabbits (GD 6 to 18) at doses up to 1000 and 300 mg/kg/day, respectively, approximately 5 and 3 times the MRHD (based on body surface area comparison). Imipenem/cilastatin was administered intravenously to mice at doses up to 320 mg/kg/day (GD 6 to 15) which is approximately equivalent to the MRHD based on body surface area comparison, and to rats at intravenous doses up to 80 mg/kg/day and a subcutaneous dose of 320 mg/kg/day (GD 6 to 17). In a separate pre-postnatal development study, rats were administered subcutaneous imipenem/cilastatin at doses up to 320 mg/kg/day (GD 15 to day 21 postpartum). The subcutaneous dose of 320 mg/kg/day in rats is approximately double the MRHD based on body surface area comparison.
Imipenem/cilastatin administered intravenously to pregnant cynomolgus monkeys during organogenesis (GD 21 to 50) at 100 mg/kg/day, a dose approximately equivalent to the MRHD (based on body surface area comparison), at an infusion rate mimicking human clinical use was not associated with fetal malformations, but there was an increase in embryonic loss relative to controls. Imipenem/cilastatin administered to pregnant cynomolgus monkeys during organogenesis at 40 mg/kg/day by bolus intravenous injection caused significant maternal toxicity including death and embryofetal loss.
Relebactam
In an embryofetal development study in pregnant mice, relebactam administered subcutaneously in doses of 80, 200, and 450 mg/kg/day during the period of organogenesis (GD 6 to 17) was not associated with maternal toxicity at doses up to 450 mg/kg/day. However, although individual skeletal malformations appeared only as single occurrences in the high dose group, the percent litter incidence of total skeletal malformations (skull and vertebral) was increased in the high-dose group (21% litter incidence) compared to the concurrent control value (5.3% litter incidence). The plasma relebactam exposure for the high dose associated with increased skeletal malformations was approximately 6 times greater than the human plasma exposure at the MRHD based on AUC comparison. Also, mice receiving the lowest administered dose of relebactam, 80 mg/kg/day, exhibited a higher percent litter incidence (15% litter incidence) of cleft palate (a rare malformation in mice) compared to the concurrent control value (0% litter incidence) and historical control values (up to 11% litter incidence). This finding did not increase in a dose-dependent manner with percent litter incidences of 0% and 5.3% in the mid- and high-dose groups respectively. The plasma AUC exposure for the low dose of relebactam associated with increased cleft palate was approximately equivalent to the human plasma AUC at the MRHD. In embryofetal development studies in rats and rabbits, intravenous relebactam was administered to rats in doses of 50, 150, and 450 mg/kg/day and rabbits in doses of 35, 275, and 450 mg/kg/day. In these studies, relebactam administered during the period of organogenesis to pregnant rats (GD 6 to 20) and rabbits (GD 7 to 20) was not associated with maternal or embryofetal toxicity at doses up to 450 mg/kg/day corresponding to plasma AUC exposures of approximately 7 and 24 times, respectively, the human plasma AUC at the MRHD.
In a pre-postnatal development study, relebactam administered intravenously in doses of 65, 200, and 450 mg/kg/day to rats from GD 6 to lactation day (LD) 20 produced no maternal toxicity and did not impair the physical and behavioral development or reproduction in first generation offspring at doses up to 450 mg/kg/day corresponding to a plasma AUC exposure of approximately 8 times the plasma AUC exposure in humans at the MRHD.
Studies in pregnant rats and rabbits showed that relebactam is transferred to the fetus through the placenta, with fetal plasma concentrations up to 5% to 6% of maternal concentrations observed on GD 20.
8.2 Lactation
Risk Summary
There are insufficient data on the presence of imipenem/cilastatin and relebactam in human milk, and no data on the effects on the breastfed child, or the effects on milk production. Relebactam is present in the milk of lactating rats (see Data).
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for RECARBRIO and any potential adverse effects on the breastfed child from RECARBRIO or from the underlying maternal condition.
8.4 Pediatric Use
The safety and efficacy of RECARBRIO in patients younger than 18 years of age have not been established.
8.5 Geriatric Use
Of the 266 patients treated with RECARBRIO in Trial 1, 113 (42.5%) were 65 years of age or older, including 55 (20.7%) patients 75 years of age and older. Of the 216 patients treated with imipenem/cilastatin plus relebactam 250 mg in Trials 2 and 3, 67 (31.0%) were 65 years of age or older, including 25 (11.6%) patients 75 years of age and older. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
RECARBRIO is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. No dosage adjustment is required based on age. Dosage adjustment for elderly patients should be based on renal function [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Reduce RECARBRIO dosage in patients with a CLcr less than 90 mL/min [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
In the event of overdose, discontinue RECARBRIO, treat symptomatically, and institute general supportive treatment. Imipenem, cilastatin, and relebactam can be removed by hemodialysis [see Clinical Pharmacology (12.3)]. No clinical information is available on the use of hemodialysis to treat overdosage.
-
11 DESCRIPTION
RECARBRIO (imipenem, cilastatin, and relebactam) for injection is an antibacterial combination product consisting of imipenem, a carbapenem antibacterial drug, cilastatin, a renal dehydropeptidase inhibitor, and relebactam, a diazabicyclooctane beta-lactamase inhibitor, for intravenous administration.
Imipenem
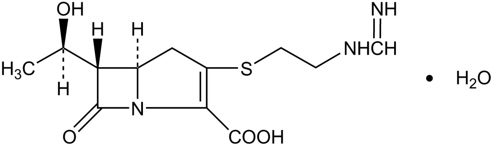
Imipenem is a beta lactam antibacterial drug. Imipenem (N-formimidoylthienamycin monohydrate) is a crystalline derivative of thienamycin, which is produced by Streptomyces cattleya. The chemical name is (5R,6S)-3-[[2-(formimidoylamino)ethyl]thio]-6-[(R)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monohydrate. It is an off-white, non-hygroscopic crystalline compound, sparingly soluble in water. The empirical formula is C12H17N3O4S∙H2O and the molecular weight is 317.37.
Figure 1: Chemical structure of imipenem
Cilastatin
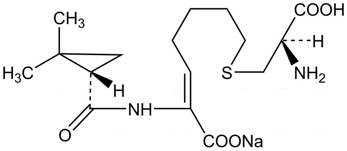
Cilastatin sodium is the sodium salt of a derivatized heptenoic acid. The chemical name is sodium (Z)-7-[[(R)-2-amino-2-carboxyethyl]thio]-2-[(S)-2,2-dimethylcyclopropanecarboxamido]-2-heptenoate. It is an off-white to white, hygroscopic, amorphous compound, very soluble in water. The empirical formula is C16H25N2NaO5S and the molecular weight is 380.44.
Figure 2: Chemical structure of cilastatin sodium
Relebactam
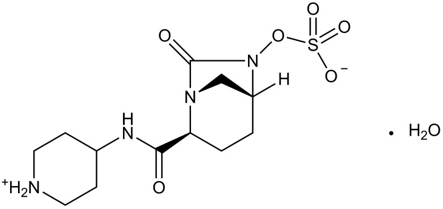
Relebactam is a beta-lactamase inhibitor. It is a crystalline monohydrate. The chemical name is (1R,2S,5R)-7-oxo-2-(piperidin-1-ium-4-ylcarbamoyl)-1,6-diazabicyclo[3.2.1]octan-6-yl sulfate hydrate. It is a white to off-white powder, soluble in water. The empirical formula is C12H20N4O6S∙H2O and the molecular weight is 366.39.
Figure 3: Chemical structure of relebactam
RECARBRIO is supplied as a white to light yellow sterile powder for constitution in a single-dose vial containing 500 mg imipenem (equivalent to 530 mg imipenem monohydrate), 500 mg cilastatin (equivalent to 531 mg cilastatin sodium), and 250 mg relebactam (equivalent to 263 mg relebactam monohydrate). Each vial of RECARBRIO is buffered with 20 mg sodium bicarbonate to provide solutions in the pH range of 6.5 to 7.6. The total sodium content of the mixture in the vial is 37.5 mg (1.6 mEq). Solutions of RECARBRIO range from colorless to yellow. Variations of color within this range do not affect the potency of the product.
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
For imipenem, the % time of dosing interval that unbound plasma concentrations of imipenem exceed the imipenem/relebactam minimum inhibitory concentration (MIC) (%fT>MIC) against the infecting organism best correlates with antibacterial activity in animal and in vitro models of infection. For relebactam the ratio of the 24-hour unbound plasma relebactam AUC to imipenem/relebactam MIC (fAUC0–24hr/MIC) best predicts the activity of relebactam in animal and in vitro models of infection.
12.3 Pharmacokinetics
The steady-state pharmacokinetic parameters of imipenem and relebactam in patients with active bacterial infection with CLcr 90 mL/min or greater following administration of the recommended dosage are summarized in Table 5.
Table 5: Population Pharmacokinetic Model-Based Steady State Mean (±SD) Plasma Pharmacokinetic Parameters of Imipenem and Relebactam After Multiple 30 Minute Intravenous Infusions* of Imipenem 500 mg/Cilastatin 500 mg and Relebactam 250 mg Every 6 Hours in Patients with CLcr 90 mL/min or Greater PK Parameters cUTI/cIAI Patients HABP/VABP Patients AUC0-24hr=area under the concentration time curve from 0 to 24 hours
Cmax=maximum concentration
CL=plasma clearance- *
- Imipenem/cilastatin and relebactam were administered either as separate infusions given concurrently or as the fixed dose combination (RECARBRIO).
Imipenem AUC0-24hr (µM-hr) 570.6 (253.3) 771 (342.3) Cmax (µM) 116.1 (52.4) 122.7 (56.8) CL (L/hr) 14 (6.1) 10.4 (4.5) Relebactam AUC0-24hr (µM-hr) 415.8 (212.6) 692.9 (354.3) Cmax (µM) 62.1 (24.7) 80 (33.3) CL (L/hr) 8.7 (4.5) 5.2 (2.7) Distribution
The binding of imipenem and cilastatin to human plasma proteins is approximately 20% and 40%, respectively. The binding of relebactam to human plasma proteins is approximately 22% and is independent of concentration at a range of 5 to 50 µM.
The penetration of imipenem and relebactam into pulmonary epithelial lining fluid is similar, with concentrations around 55% and 54% of unbound plasma concentrations of imipenem and relebactam, respectively.
The steady-state volume of distribution of imipenem, cilastatin, and relebactam is 24.3 L, 13.8 L, and 19.0 L, respectively, in subjects following multiple doses infused over 30 minutes every 6 hours.
Elimination
Imipenem and relebactam are eliminated from the body by the kidneys with a mean (±SD) half-life of 1 (±0.5) hour and 1.2 (±0.7) hours, respectively.
Metabolism
Imipenem, when administered alone, is metabolized in the kidneys by dehydropeptidase, resulting in low levels of imipenem recovered in human urine. Cilastatin, an inhibitor of this enzyme, effectively prevents renal metabolism so that when imipenem and cilastatin are given concomitantly, adequate concentrations of imipenem are achieved in the urine to enable antibacterial activity.
Relebactam is minimally metabolized. Unchanged relebactam was the only drug-related component detected in human plasma.
Excretion
Imipenem, cilastatin, and relebactam are mainly excreted by the kidneys.
Following multiple-dose administration of imipenem 500 mg, cilastatin 500 mg, and relebactam 250 mg to healthy male subjects, approximately 63% of the administered imipenem dose, and 77% of the administered cilastatin dose are recovered as unchanged parent drugs in the urine. The renal excretion of imipenem and cilastatin involves both glomerular filtration and active tubular secretion. Greater than 90% of the administered relebactam dose was excreted unchanged in human urine. The unbound renal clearance of relebactam is greater than the glomerular filtration rate, suggesting that in addition to glomerular filtration, active tubular secretion is involved in the renal elimination, accounting for ~30% of the total clearance.
Specific Populations
No clinically significant differences in the pharmacokinetics of imipenem, cilastatin, or relebactam were observed based on age, gender, or race/ethnicity.
Patients with Renal Impairment
In a single-dose trial evaluating the effect of renal impairment on the PK of relebactam 125 mg co-infused with imipenem/cilastatin 250 mg (half the recommended dose in patients with normal renal function), mean AUC was higher in subjects with CLcr 60-89, 30-59, and 15-29 mL/min, respectively, compared to healthy subjects with CLcr 90 mL/min or greater (Table 6). In subjects with end stage renal disease (ESRD) on hemodialysis, imipenem, cilastatin, and relebactam are removed by hemodialysis, with extraction coefficients of 66% to 87% for imipenem, 46% to 56% for cilastatin, and 67% to 87% for relebactam.
Table 6: Mean AUC Increase in Subjects with Renal Impairment Compared to Subjects with CLcr 90 mL/min or Greater Estimated CLcr (mL/min) Imipenem Cilastatin Relebactam 60 to 89 1.1-fold 1.2-fold 1.2-fold 30 to 59 1.7-fold 2.0-fold 2.2-fold 15 to 29 2.6-fold 5.5-fold 4.7-fold To maintain systemic exposures similar to patients with normal renal function, dose adjustment is recommended for patients with renal impairment [see Dosage and Administration (2.2)]. ESRD patients on hemodialysis should receive RECARBRIO after hemodialysis session [see Dosage and Administration (2.2)].
Drug Interaction Studies
Clinical Studies
No drug-drug interaction was observed among imipenem, cilastatin, and relebactam in a clinical study in healthy subjects.
No clinically significant differences in the pharmacokinetics of imipenem or relebactam were observed when RECARBRIO was used concomitantly with probenecid (Organic Anion Transporter 3 (OAT3) inhibitor).
In Vitro Studies
CYP Enzymes
Relebactam does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A4 or induce CYP1A2, CYP2B6, or CYP3A4 in human hepatocytes.
Transporter Systems
Relebactam does not inhibit OATP1B1, OATP1B3, OAT1, OAT3, OCT2, P-gp, BCRP, MATE1, MATE2K, or BSEP.
Relebactam is not a substrate of OAT1, OCT2, P-gp, BCRP, MRP2, or MRP4 transporters, but is a substrate of OAT3, OAT4, MATE1, and MATE2K transporters.
The following antibacterial and antifungal drugs (piperacillin/tazobactam, ciprofloxacin, fluconazole, ampicillin, levofloxacin, metronidazole, vancomycin, linezolid, daptomycin, and cefazolin) did not significantly inhibit OAT3-mediated relebactam uptake.
12.4 Microbiology
Mechanism of Action
RECARBRIO is a combination of imipenem/cilastatin and relebactam. Imipenem is a penem antibacterial drug, cilastatin sodium is a renal dehydropeptidase inhibitor, and relebactam is a beta-lactamase inhibitor. Cilastatin limits the renal metabolism of imipenem and does not have antibacterial activity. The bactericidal activity of imipenem results from binding to PBP 2 and PBP 1B in Enterobacteriaceae and Pseudomonas aeruginosa and the subsequent inhibition of penicillin binding proteins (PBPs). Inhibition of PBPs leads to the disruption of bacterial cell wall synthesis. Imipenem is stable in the presence of some beta-lactamases. Relebactam has no intrinsic antibacterial activity. Relebactam protects imipenem from degradation by certain serine beta-lactamases such as Sulfhydryl Variable (SHV), Temoneira (TEM), Cefotaximase-Munich (CTX-M), Enterobacter cloacae P99 (P99), Pseudomonas-derived cephalosporinase (PDC, AmpC-type), and Klebsiella-pneumoniae carbapenemase (KPC).
Resistance
Clinical isolates may produce multiple beta-lactamases, express varying levels of beta-lactamases, have amino acid sequence variations, or have other resistance mechanisms that have not yet been identified. Culture and susceptibility information and local epidemiology should be considered in selecting or modifying antibacterial therapy.
Mechanisms of beta lactam resistance in gram-negative organisms include the production of beta-lactamases, up-regulation of efflux pumps, and loss of outer membrane porins. Imipenem/relebactam retains activity in the presence of the tested efflux pumps. Imipenem/relebactam has shown activity against some isolates of P. aeruginosa and Enterobacteriaceae that produce relebactam-susceptible beta-lactamases concomitant with loss of entry porins. Imipenem/relebactam is not active against most isolates containing metallo-beta-lactamases (MBLs), some oxacillinases with carbapenemase activity, as well as certain alleles of GES.
Imipenem/relebactam demonstrated in vitro activity against some Enterobacteriaceae isolates genotypically characterized for some beta-lactamases and extended-spectrum beta-lactamases (ESBLs) of the following groups: KPC, TEM, SHV, CTX-M, CMY, DHA, and ACT/MIR. Many of the Enterobacteriaceae isolates that were not susceptible to imipenem-relebactam were genotypically characterized and the genes encoding MBLs or certain oxacillinases were present.
Imipenem/relebactam demonstrated in vitro activity against genotypically characterized P. aeruginosa isolates containing certain known resistance factors: some chromosomal PDC alleles with ESBLs, and some with loss of outer membrane porin (OprD) with or without co-expression of up-regulated efflux pumps (MexAB, MexCD, MexJK, and MexXY). Genotypically characterized P. aeruginosa isolates that were not susceptible to imipenem/relebactam encoded some MBL, KPC, PER, GES, VEB, and PDC alleles.
Methicillin-resistant staphylococci should be considered resistant to imipenem. Imipenem is inactive in vitro against Enterococcus faecium, Stenotrophomonas maltophilia, and some isolates of Burkholderia cepacia.
No cross-resistance with other classes of antimicrobials has been identified. Some isolates resistant to carbapenems (including imipenem) and to cephalosporins may be susceptible to RECARBRIO.
Interaction with Other Antimicrobials
In vitro studies have demonstrated no antagonism between imipenem/relebactam and amikacin, azithromycin, aztreonam, colistin, gentamicin, levofloxacin, linezolid, tigecycline, tobramycin, or vancomycin.
Activity against Imipenem-Nonsusceptible Bacteria in Animal Infection Models
Relebactam restored activity of imipenem/cilastatin in animal models of infection (e.g., mouse disseminated infection, mouse thigh infection, and mouse pulmonary infection) caused by imipenem-nonsusceptible KPC-producing Enterobacteriaceae and imipenem-nonsusceptible P. aeruginosa (imipenem-nonsusceptible due to production of chromosomal PDC and loss of OprD porin).
Antimicrobial Activity
RECARBRIO has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections [see Indications and Usage (1.1) and (1.2)].
Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP)
- Aerobic Bacteria
- Gram-negative Bacteria
- Acinetobacter calcoaceticus-baumannii complex
- Enterobacter cloacae
- Escherichia coli
- Haemophilus influenzae
- Klebsiella aerogenes
- Klebsiella oxytoca
- Klebsiella pneumoniae
- Pseudomonas aeruginosa
- Serratia marcescens
Complicated Urinary Tract Infections (cUTI)
- Aerobic Bacteria
- Gram-negative Bacteria
- Klebsiella aerogenes
- Enterobacter cloacae
- Escherichia coli
- Klebsiella pneumoniae
- Pseudomonas aeruginosa
Complicated Intra-abdominal Infections (cIAI)
- Aerobic Bacteria
- Gram-negative Bacteria
- Citrobacter freundii
- Klebsiella aerogenes
- Enterobacter cloacae
- Escherichia coli
- Klebsiella oxytoca
- Klebsiella pneumoniae
- Pseudomonas aeruginosa
- Anaerobic Bacteria
- Gram-negative Bacteria
- Bacteroides caccae
- Bacteroides fragilis
- Bacteroides ovatus
- Bacteroides stercoris
- Bacteroides thetaiotaomicron
- Bacteroides uniformis
- Bacteroides vulgatus
- Fusobacterium nucleatum
- Parabacteroides distasonis
The following in vitro data are available, but their clinical significance is unknown. At least 90% of the following bacteria exhibit an in vitro MIC less than or equal to the susceptible breakpoint for RECARBRIO against isolates of similar genus or organism group. However, the efficacy of RECARBRIO in treating clinical infections due to these bacteria has not been established in adequate and well-controlled clinical trials.
- Aerobic Bacteria
- Gram-positive Bacteria
- Enterococcus faecalis
- Methicillin-susceptible Staphylococcus aureus
- Streptococcus anginosus
- Streptococcus constellatus
- Streptococcus pneumoniae
- Gram-negative Bacteria
- Citrobacter koseri
- Enterobacter asburiae
- Anaerobic Bacteria
- Gram-positive Bacteria
- Eggerthella lenta
- Parvimonas micra
- Peptoniphilus harei
- Peptostreptococcus anaerobius
- Gram-negative Bacteria
- Fusobacterium necrophorum
- Fusobacterium varium
- Parabacteroides goldsteinii
- Parabacteroides merdae
- Prevotella bivia
- Veillonella parvula
Susceptibility Test Methods
For specific information regarding susceptibility testing methods, interpretive criteria, and associated test methods and quality control standards recognized by FDA for RECARBRIO, please see: https://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies with imipenem/cilastatin or relebactam have not been conducted.
Mutagenesis
Genotoxicity studies were performed in a variety of bacterial and mammalian tests in vivo and in vitro. None of these tests showed any evidence of genetic damage.
The tests conducted with imipenem, cilastatin, or imipenem/cilastatin included: V79 mammalian cell mutagenesis assay (imipenem, cilastatin), Ames test (imipenem, cilastatin), unscheduled DNA synthesis assay (imipenem/cilastatin), and in vivo mouse cytogenetics test (imipenem/cilastatin).
The tests conducted with relebactam included: Ames test, in vitro chromosomal aberration in Chinese Hamsters Ovary (CHO) cells, and in vivo rat micronucleus test.
Impairment of Fertility
No adverse effects on fertility, reproductive performance, fetal viability, growth or postnatal development were observed in male and female rats given imipenem/cilastatin at intravenous doses up to 80 mg/kg/day and at a subcutaneous dose of 320 mg/kg/day. In rats, a dose of 320 mg/kg was approximately double the MRHD based on body surface area. Slight decreases in live fetal body weight were restricted to the highest dosage level.
In fertility studies, relebactam was administered in intravenous doses of 50, 150, and 450 mg/kg/day to male rats beginning 15 days before mating, through mating, and for an additional 3 weeks and to female rats beginning 15 days before mating, through mating, and until gestation day (GD) 7. Relebactam did not impair fertility, reproductive performance or spermatogenesis in males or fertility, reproductive performance, or early embryonic development in females at doses up to 450 mg/kg/day corresponding to plasma AUC exposures of approximately 8 times in males and 7 times in females the plasma AUC exposure in humans at the MRHD.
13.2 Animal Toxicology and/or Pharmacology
Relebactam given as a single entity caused renal tubular degeneration in monkeys at AUC exposure 7-fold the human AUC exposure at the MRHD. Renal tubular degeneration was shown to be reversible after dose discontinuation. There was no evidence of nephrotoxicity at AUC exposures less than or equal to 3-fold the human AUC exposure at the MRHD.
-
14 CLINICAL STUDIES
14.1 Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia
A total of 535 hospitalized adults with HABP/VABP were randomized and received trial medications in a multinational, double-blind trial (Trial 1, NCT02493764) comparing RECARBRIO 1.25 grams (imipenem 500 mg/cilastatin 500 mg/relebactam 250 mg) intravenously every 6 hours to piperacillin and tazobactam (4.5 grams) for 7 to 14 days of therapy.
The modified intent-to-treat (MITT) population, which included all randomized patients who received at least one dose of trial treatment and did not have only gram-positive cocci on Gram stain of the baseline lower respiratory tract (LRT) specimen included 531 patients; the mean age was 60 and 43% were 65 years of age or older. The majority of patients were men (69%), white (78%), and from Europe (61%). The mean APACHE II score was 15 and 47% of the population had an APACHE II score of ≥15. At randomization, 66% of patients were admitted to the ICU, 77% had been in the hospital for ≥5 days, and 48% had a creatinine clearance of <90 mL/min. Concurrent bacteremia was present at baseline in 5.8% of patients.
Table 7 presents the incidence of all-cause mortality through Day 28 and clinical response at the early follow-up (EFU) visit (7 to 14 days after the end of therapy) in the MITT population. Overall results are presented along with subgroup results by pneumonia diagnosis.
Table 7: Day 28 All-Cause Mortality and Clinical Response Rates at EFU from Trial 1 of Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP) (MITT Population) RECARBRIO Piperacillin/Tazobactam Treatment Difference n/m (%) n/m (%) %* (95% CI)* EFU = early follow up - *
- Treatment differences and 95% confidence intervals are based on Miettinen & Nurminen method.
- †
- n/m = number of subjects with survival status of death or unknown / number of modified intent-to-treat subjects.
- ‡
- One subject in the RECARBRIO arm had unknown mortality status at Day 28 which was counted as a death.
- §
- n/m = number of subjects with a favorable clinical response / number of modified intent-to-treat subjects.
All-Cause Mortality Through Day 28†,‡ 42/264 (15.9) 57/267 (21.3) -5.3 (-11.9, 1.2) Non-ventilated HABP 18/142 (12.7) 15/131 (11.5) 1.2 (-6.8, 9.1) Ventilated HABP/VABP 24/122 (19.7) 42/136 (30.9) -11.2 (-21.6, -0.5) Clinical Response at EFU§ 161/264 (61.0) 149/267 (55.8) 5.0 (-3.2, 13.2) Non-ventilated HABP 95/142 (66.9) 87/131 (66.4) 0.5 (-10.7, 11.7) Ventilated HABP/VABP 66/122 (54.1) 62/136 (45.6) 8.5 (-3.7, 20.5) In the MITT population, in patients with an APACHE II score <15, Day 28 all-cause mortality rates were 17/139 (12.2%) for RECARBRIO-treated patients and 12/140 (8.6%) for piperacillin/tazobactam-treated patients, clinical cure rates were 90/139 (64.7%) and 98/140 (70%), respectively. In patients with an APACHE II score ≥15, Day 28 all-cause mortality rates were 25/125 (20%) for RECARBRIO-treated patients and 45/127 (35.4%) for piperacillin/tazobactam-treated patients, clinical cure rates were 71/125 (56.8%) and 51/127 (40.2%), respectively.
Per pathogen favorable clinical response at EFU and Day 28 all-cause mortality were assessed in a microbiological modified intention to treat (mMITT) population, which consisted of all randomized MITT subjects who had at least one baseline LRT pathogen that was susceptible to both study treatments (Table 8).
Table 8: Day 28 All-Cause Mortality and Favorable Clinical Response at EFU by Baseline LRT Pathogen from Trial 1 of Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP) (mMITT Population) Baseline LRT
PathogenDay 28 All-Cause Mortality Clinical Response at EFU RECARBRIO
n/m* (%)Piperacillin/
Tazobactam
n/m* (%)RECARBRIO
n/m† (%)Piperacillin/
Tazobactam
n/m† (%)LRT = lower respiratory tract
EFU = early follow-up- *
- n/m = the number of subjects with survival status of death or unknown within each category / the number of microbiological modified intent-to-treat subjects who have the corresponding baseline pathogen from LRT culture.
- †
- n/m = the number of subjects with a favorable clinical response within each category / the number of microbiological modified intent-to-treat subjects who have the corresponding baseline pathogen from LRT culture.
- ‡
- Supportive evidence was derived from the imipenem and cilastatin prescribing information.
- §
- All H. influenzae isolates were susceptible to imipenem. The susceptible MIC breakpoint for PIP/TAZ is ≤1/4 mcg/mL. At the lowest concentration of PIP/TAZ tested (2/4 mcg/mL) there was no visible growth.
- ¶
- Includes Klebsiella aerogenes, Klebsiella oxytoca, Klebsiella pneumoniae.
Acinetobacter
calcoaceticus-
baumannii complex0/5‡ (0.0) 1/10 (10.0) 4/5‡ (80.0) 6/10 (60.0) Enterobacter
cloacae1/7‡ (14.3) 3/16 (18.8) 6/7‡ (85.7) 12/16 (75.0) Escherichia coli 5/27(18.5) 8/33 (24.2) 16/27 (59.3) 19/33 (57.6) Haemophilus
influenzae§2/13 (15.4) 3/12 (25.0) 9/13 (69.2) 8/12 (66.7) Klebsiella spp.¶ 6/42 (14.3) 8/41 (19.5) 25/42 (59.5) 28/41 (68.3) Pseudomonas
aeruginosa7/26 (26.9) 5/35 (14.3) 12/26 (46.2) 20/35 (57.1) Serratia
marcescens2/10 (20.0) 1/4 (25.0) 7/10 (70.0) 3/4 (75.0) 14.2 Complicated Urinary Tract Infections, including Pyelonephritis and Complicated Intra-abdominal Infections
The determination of efficacy and safety of RECARBRIO was supported in part by the previous findings of the efficacy and safety of imipenem/cilastatin for the treatment of cUTI and cIAI. The contribution of relebactam to RECARBRIO was primarily established in vitro and in animal models of infection [see Microbiology (12.4)]. Imipenem/cilastatin plus relebactam was studied in cUTI including pyelonephritis (Trial 2, NCT01505634) and cIAI (Trial 3, NCT01506271) in randomized, blinded, active-controlled, multicenter trials. These trials provided only limited efficacy and safety information.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
RECARBRIO (imipenem, cilastatin, and relebactam) for injection, 1.25 grams is supplied as a white to light yellow sterile powder for constitution in a single-dose glass vial containing imipenem 500 mg (equivalent to 530 mg imipenem monohydrate), cilastatin 500 mg (equivalent to 531 mg cilastatin sodium), and relebactam 250 mg (equivalent to 263 mg relebactam monohydrate).
The vials are supplied as a single-dose glass vial (NDC 0006-3856-01) and in cartons containing 25 vials (NDC 0006-3856-02).
-
17 PATIENT COUNSELING INFORMATION
Serious Allergic Reactions
Advise patients, their families, or caregivers that allergic reactions, including serious allergic reactions, could occur that require immediate treatment. Ask them about any previous hypersensitivity reactions to RECARBRIO (imipenem, cilastatin, and relebactam), carbapenems, penicillins, cephalosporins, other beta lactams, or other allergens [see Warnings and Precautions (5.1)].
Seizures and Central Nervous System Reactions
Counsel patients, their families, or caregivers to inform a healthcare provider if they have central nervous system disorders, such as stroke or history of seizures. Seizures have been reported during treatment with imipenem, a component of RECARBRIO, especially when recommended dosages were exceeded and with closely related antibacterial drugs [see Warnings and Precautions (5.2)].
Drug Interaction with Valproic Acid
Counsel patients, their families, or caregivers to inform a healthcare provider if they are taking valproic acid or divalproex sodium. If treatment with RECARBRIO is necessary, supplemental anti-convulsant medication to prevent and/or treat seizures may be needed [see Warnings and Precautions (5.3)].
Potentially Serious Diarrhea
Advise patients, their families, or caregivers that diarrhea is a common problem caused by antibacterial drugs, including RECARBRIO and usually resolves when the drug is discontinued. Sometimes, frequent watery or bloody diarrhea may occur and may be a sign of a more serious intestinal infection that may require treatment. If severe watery or bloody diarrhea develops, tell the patient to contact his or her healthcare provider [see Warnings and Precautions (5.4)].
Antibacterial Resistance
Patients should be counseled that antibacterial drugs, including RECARBRIO, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When RECARBRIO is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by RECARBRIO or other antibacterial drugs in the future [see Warnings and Precautions (5.5)].
-
SPL UNCLASSIFIED SECTION
Merck Sharp & Dohme LLC
Rahway, NJ 07065, USAFor patent information: www.msd.com/research/patent
Copyright © 2019-2022 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.uspi-mk7655a-iv-2205r003
-
PRINCIPAL DISPLAY PANEL - 1.25 g Vial Carton Label
NDC 0006-3856-02
Recarbrio™
(imipenem, cilastatin, and relebactam)
for Injection
1.25 g per vial*Must be constituted and further diluted.
See enclosed package insert for preparation instructions.For Intravenous Infusion Only
*Each vial contains imipenem 500 mg (equivalent to 530 mg imipenem monohydrate), cilastatin 500 mg (equivalent
to 531 mg cilastatin sodium), and relebactam 250 mg (equivalent to 263 mg relebactam monohydrate).
Inactive ingredient: 20 mg sodium bicarbonate added to each vial as a buffer.25 single-dose vials
Rx only

-
INGREDIENTS AND APPEARANCE
RECARBRIO
imipenem anhydrous, cilastatin, and relebactam anhydrous injection, powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0006-3856 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IMIPENEM ANHYDROUS (UNII: Q20IM7HE75) (IMIPENEM ANHYDROUS - UNII:Q20IM7HE75) IMIPENEM ANHYDROUS 500 mg in 100 mL CILASTATIN (UNII: 141A6AMN38) (CILASTATIN - UNII:141A6AMN38) CILASTATIN 500 mg in 100 mL RELEBACTAM ANHYDROUS (UNII: 1OQF7TT3PF) (RELEBACTAM ANHYDROUS - UNII:1OQF7TT3PF) RELEBACTAM ANHYDROUS 250 mg in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) 20 mg in 100 mL Product Characteristics Color WHITE (white to light yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0006-3856-02 25 in 1 CARTON 01/06/2020 1 NDC:0006-3856-01 20 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212819 01/06/2020 Labeler - Merck Sharp & Dohme LLC (118446553)

