Label: CISPLATIN injection, solution
- NDC Code(s): 60505-6277-0
- Packager: Apotex Corp.
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
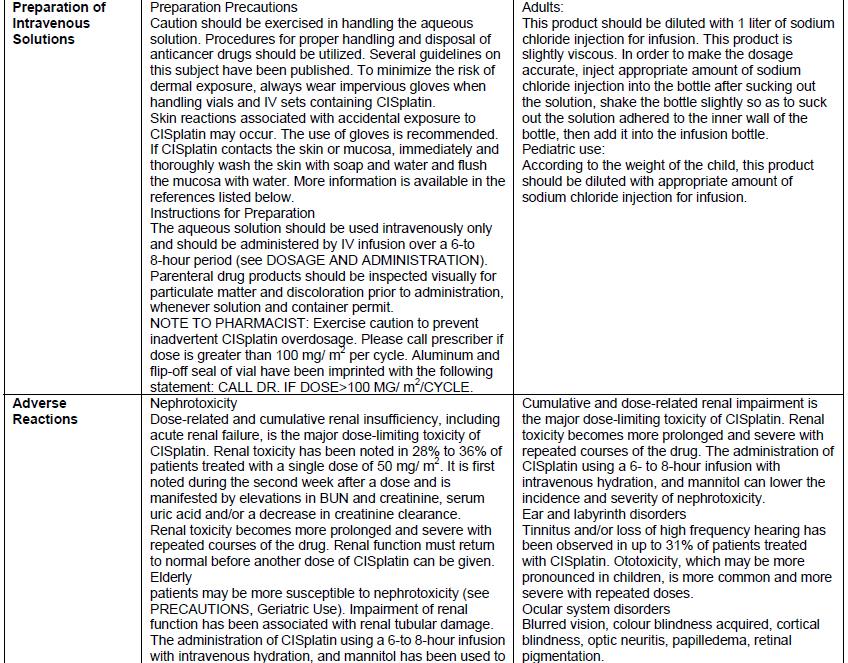
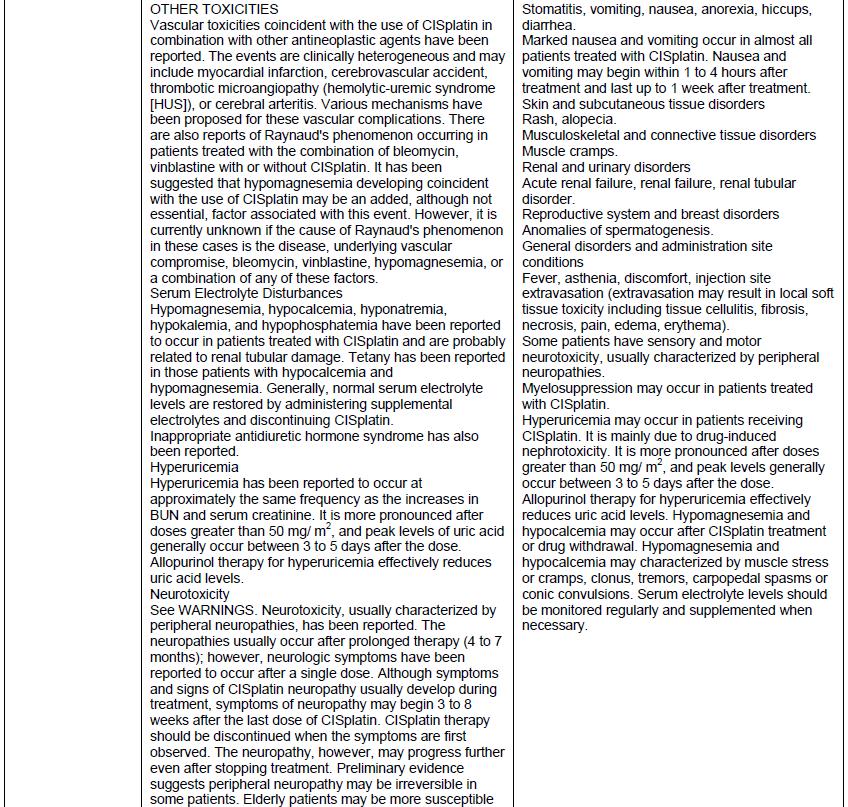
Drug Label Information
Updated December 21, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HEALTH CARE PROVIDER LETTER
IMPORTANT PRESCRIBING INFORMATION
December 6, 2023
Subject: Temporary Importation of CISplatin Injection (50 mg/50 mL) with non-U.S. Labeling to Address Drug Shortage
Dear Healthcare Professional,
Due to the critical shortage of CISplatin Injection in the United States (U.S.), Qilu Pharmaceutical Co. Ltd (Qilu), in conjunction with Apotex Corp., is coordinating with the U.S. Food and Drug Administration (FDA) to increase the availability of the drug. Qilu has initiated temporary importation of CISplatin Injection (50 mg/50 mL) with vial and carton labels in Chinese into the U.S. market. The CISplatin Injection from Qilu is marketed and manufactured in China and is not FDA-approved.
Only Qilu or its distributor, Apotex Corp., is authorized by the FDA to import or distribute Qilu's CISplatin Injection in the United States.
Effective immediately and during this temporary period, Apotex Corp. will distribute the following presentation of CISplatin Injection to address the critical shortage:
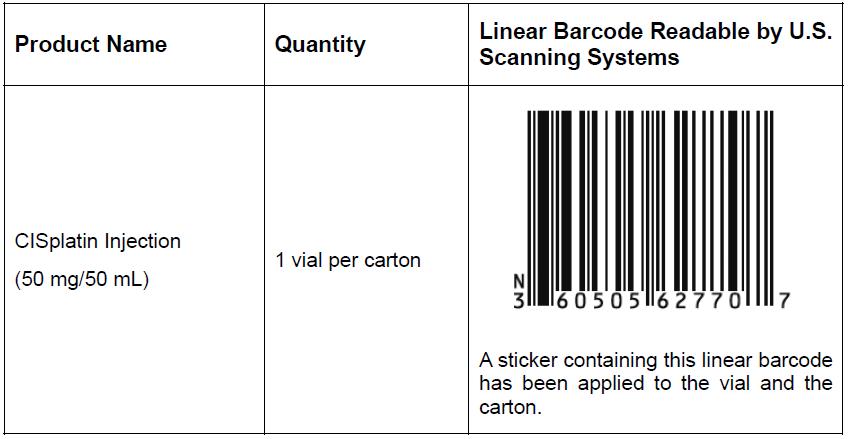
Product Name Quantity Description U.S. NDC Number Lot Number Expiration Date CISplatin Injection (50 mg/50 mL) 1 vial per carton Colorless to yellowish clear liquid
Each 1 mL contains 1 mg of CISplatin and 9 mg of Sodium Chloride in water for injection.60505-6277-0
See Appendix 1 for a scannable linear barcode readable by U.S. scanning systems.3J025C88 2025-09-26 3J026C88 2025-09-26 3J027C88 2025-09-26 3K028C88 2025-10-08 3K029C88 2025-10-08 3K030C88 2025-10-08 3K031C88 2025-10-09 3K032C88 2025-10-09 3K033C88 2025-10-09 3K034C88 2025-10-11 3K035C88 2025-10-11 3K036C88 2025-10-11 It is important to note the following:
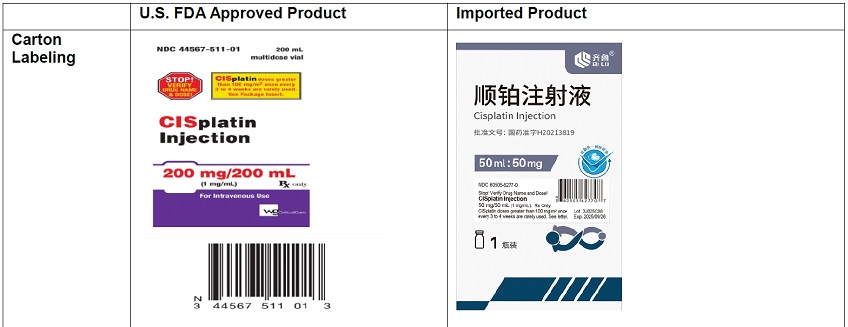
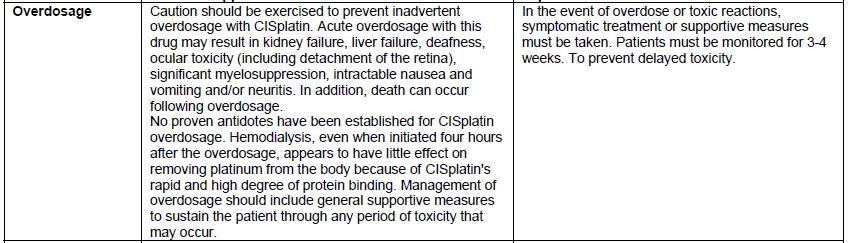
- The carton labeling and vial label did not include the warning statements, “Stop! Verify Drug Name and Dose!” or “CISplatin doses greater than 100 mg/m 2once every 3 to 4 weeks are rarely used”. Thus, a sticker containing this warning statement, the name of the product, strength, concentration, U.S. NDC number, Lot number, expiration date, Rx only, and linear barcode has been applied to the vial and the carton.
- The vial label did not have the translated name of the product “CISplatin”. Thus, a sticker containing the information noted in the bullet above has been applied to the vial.
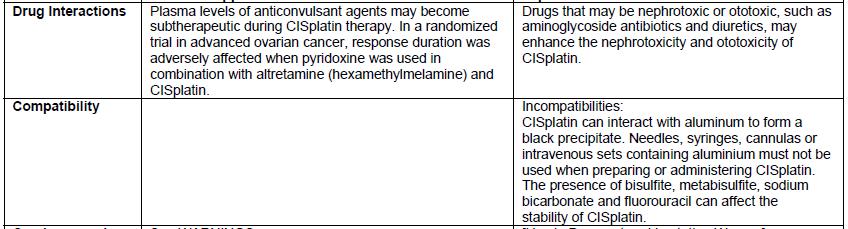
- Incompatible with solutions containing bisulfite, metabisulfite, sodium bicarbonate and fluorouracil.
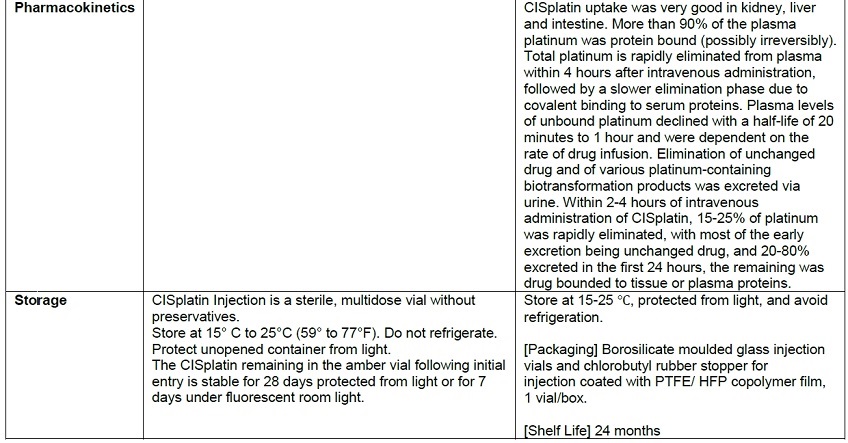
- The product is colorless to yellowish clear liquid.
- The vial and carton labels will display the text used and approved for marketing the products in China containing Chinese only text. Example images of this labeling are provided in Appendix 2.
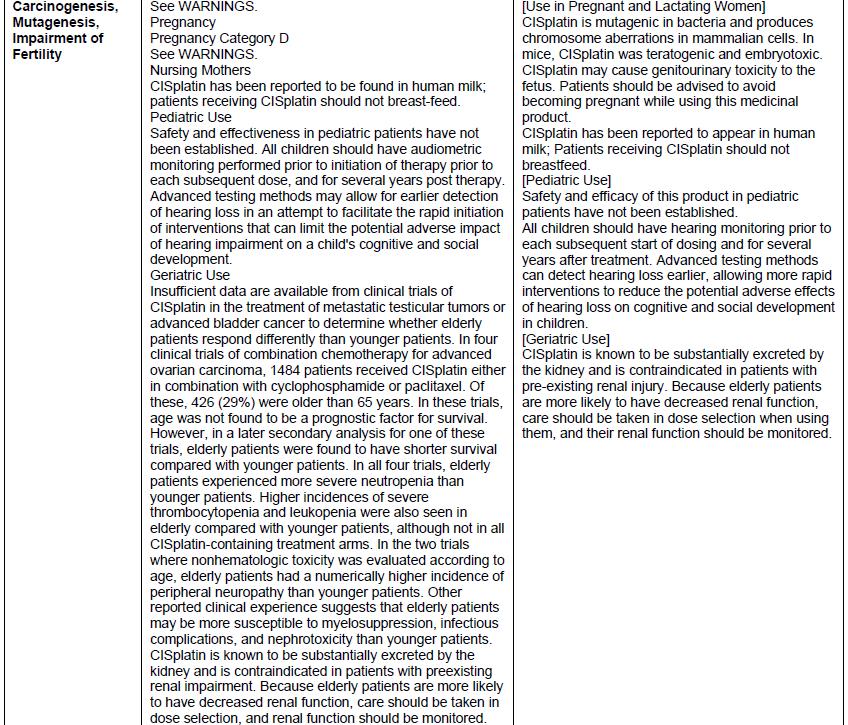
- There are differences in the format and content of the labeling between the FDA-approved product and Qilu’s CISplatin Injection. Please see the product comparison table in Appendix 3 and corresponding English translations.
- The labeling for the imported product states that this product is slightly viscous and to achieve an accurate dose, you might need to rinse the vial with sodium chloride injection to remove the solution adhered to the inner wall of the vial.
CISplatin injection is available only by prescription in the U.S. The imported lots did not have the statement “Rx only” on their labeling. This information is included on the sticker noted in the bullet above.
The carton of the imported product does not include a product identifier. Specifically, each package of product does not include the NDC, unique serial number, lot number, and expiration date in both human-readable form and a two-dimensional data matrix barcode.
Please refer to the package insert for the FDA-approved CISplatin Injection drug product for full prescribing information.
Finally, please ensure that your staff and others in your institution who may be involved in the administration of CISplatin Injection receive a copy of this letter and review the information.
If you have any questionsabout the information contained in this letter, any quality related problems, or questions on the use of Qilu’s CISplatin Injection, please contact Apotex Corp. Customer Service at 1-800-706-5575.
For ordering information, please contact your primary wholesaler or distributor to place an order with Apotex Corp. at 1-800-706-5575.
Healthcare providers should report adverse events associated with the use of Qilu’s CISplatin Injection to Apotex Corp. at 1-800-706-5575.
Adverse events or quality problems experienced with the use of this product may also be reported to the FDA’s MedWatch Adverse Event Reporting Program either online, by regular mail, or by fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178 (1-800-332-0178).
We remain at your disposal to answer any questions you may have about our product; and provide more information if needed.
Sincerely,
Mr. Yin Xunliao
Deputy General Manager
Qilu Pharmaceutical Co., Ltd.Enclosures:
Appendix 1 – Barcodes for Pharmacy Dispensing
Appendix 2 – Product Label and Product Characteristics Side-by-Side Comparison Table
Appendix 3 – Prescribing Information Side-by-Side Comparison Table
Available at www1.apotex.com/us/CISplatin_InjectionAppendix 1: Barcode for Pharmacy Dispensing
Appendix 2: Product Label and Product Characteristics Side-by-Side Comparison Table
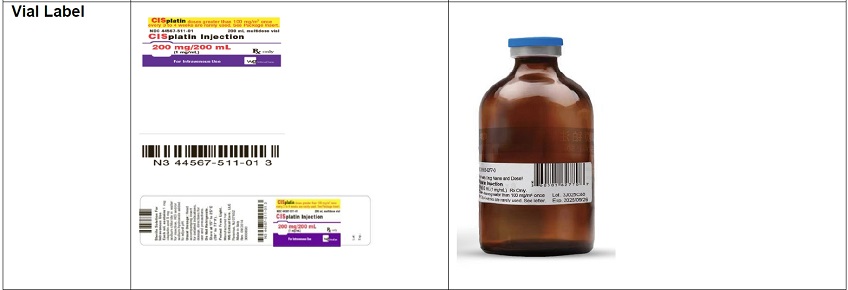
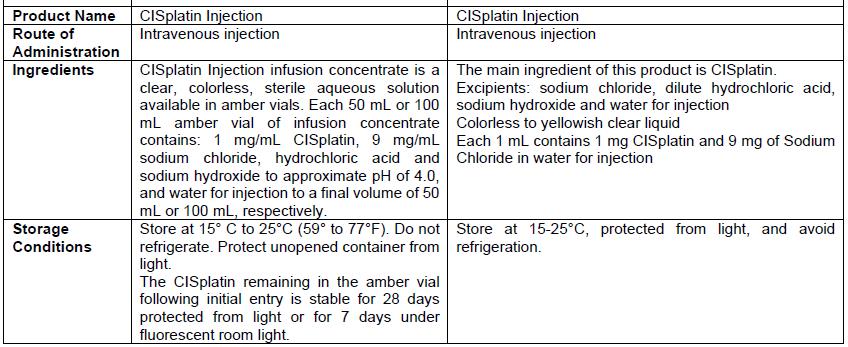
Appendix 3: Prescribing Information Side-by-Side Comparison Table (translated from Chinese)
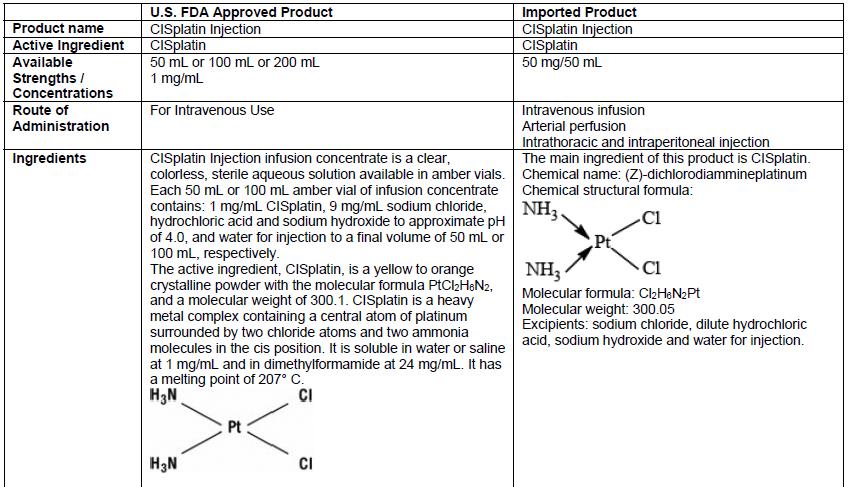



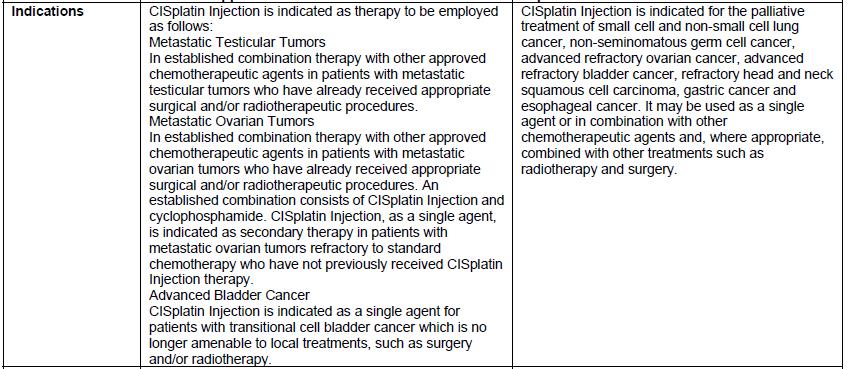

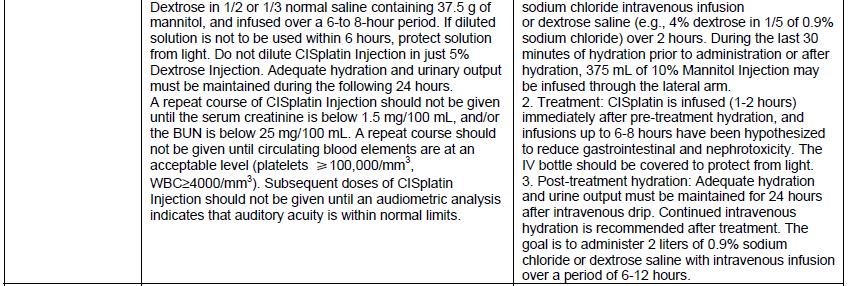











DHCP version: 34040099636B
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CISPLATIN
cisplatin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:60505-6277 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CISPLATIN (UNII: Q20Q21Q62J) (CISPLATIN - UNII:Q20Q21Q62J) CISPLATIN 50 mg in 50 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60505-6277-0 1 in 1 CARTON 06/06/2023 1 50 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 06/06/2023 Labeler - Apotex Corp. (845263701) Establishment Name Address ID/FEI Business Operations Qilu Pharmaceutical Co., Ltd. (Biological Industrial Park) 544532200 manufacture(60505-6277) , analysis(60505-6277) , pack(60505-6277)




