Label: DEEP BLUE COPAIBA- menthol stick
-
Contains inactivated NDC Code(s)
NDC Code(s): 71630-882-48 - Packager: dōTERRA International, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 15, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
For external use only
Allergy alert: Do not use if allergic to
salicylates (including aspirin) without
consulting a physician.
When using this product • use only as
directed • avoid getting into the eyes or
on mucous membranes. If contact occurs,
rinse thoroughly with water. • do not apply to
wounds or damaged skin • do not bandage
tightly or use with a heating pad
- INSTRUCTIONS FOR USE
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients Cocos nucifera
(Coconut) Oil, Tapioca Starch, Gaultheria
procumbens (Wintergreen) Leaf Oil, Helianthus
annuus (Sunflower) Seed Wax, Copaifera
coriacea/langsdorffii/officinalis/reticulata Resin
Oil, Water (Aqua), Sambucus nigra Fruit Extract,
Prunus amygdalus dulcis (Sweet Almond) Oil,
Glyceryl Laurate, Glyceryl Caprylate, Mentha
piperita (Peppermint) Oil, Cinnamomum
camphora (Camphor) Oil, Eucalyptus globulus
Leaf Oil, Tanacetum annuum Flower/Leaf/ Stem
Oil, Glyceryl Undecylenate, Cananga odorata
Flower Oil, Capsicum frutescens Fruit Extract,
Glycerin, Lauric Acid, Chamomilla recutita
(Matricaria) Oil, Helichrysum italicum
Flower/Leaf/Stem Oil, Citric Acid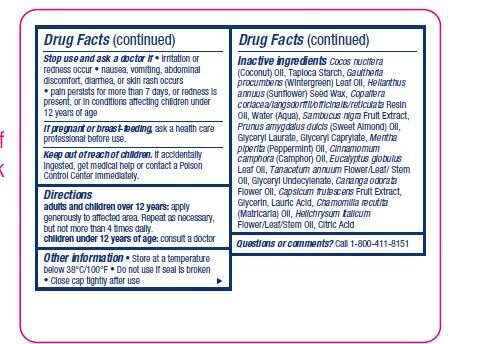
- QUESTIONS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DEEP BLUE COPAIBA
menthol stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71630-882 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 10 g in 100 g Inactive Ingredients Ingredient Name Strength COCONUT OIL (UNII: Q9L0O73W7L) STARCH, TAPIOCA (UNII: 24SC3U704I) GAULTHERIA PROCUMBENS LEAF (UNII: 2125M16OWN) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) HELICHRYSUM ITALICUM FLOWER OIL (UNII: O97ZV7726K) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ALMOND OIL (UNII: 66YXD4DKO9) PEPPERMINT OIL (UNII: AV092KU4JH) CAMPHOR OIL (UNII: 75IZZ8Y727) EUCALYPTUS OIL (UNII: 2R04ONI662) MATRICARIA CHAMOMILLA FLOWERING TOP OIL (UNII: SA8AR2W4ER) GLYCERIN (UNII: PDC6A3C0OX) CANANGA OIL (UNII: 8YOY78GNNX) SAMBUCUS NIGRA SUBSP. CERULEA FRUIT (UNII: EPM36Z6E4L) GLYCERYL LAURATE (UNII: Y98611C087) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) TANACETUM ANNUUM FLOWERING TOP OIL (UNII: E2Q02N1ZC7) GLYCERYL 1-UNDECYLENATE (UNII: B68LJT9544) TABASCO PEPPER (UNII: J1M3NA843L) LAURIC ACID (UNII: 1160N9NU9U) WATER (UNII: 059QF0KO0R) COPAIBA OIL (UNII: 64VX45Y68N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71630-882-48 48 g in 1 CYLINDER; Type 0: Not a Combination Product 07/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 07/15/2021 Labeler - dōTERRA International, LLC (832274935)

