Label: FLEXBUMIN- albumin human solution
- NDC Code(s): 0944-0493-01, 0944-0493-02, 0944-0493-03, 0944-0493-04
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FLEXBUMIN 25% safely and effectively. See full prescribing information for FLEXBUMIN 25%.
FLEXBUMIN 25% Albumin (Human), USP, 25% Solution,
for intravenous use
Initial U.S. Approval: 2005INDICATIONS AND USAGE
FLEXBUMIN 25%, Albumin (Human) Solution is indicated for:
- Hypovolemia (1.1)
- Hypoalbuminemia: Burns, Adult Respiratory Distress Syndrome (ARDS) and Nephrosis (1.2)
- Cardiopulmonary Bypass Surgery (1.3)
- Hemolytic Disease of the Newborn (HDN) (1.4)
Limitations of Use: Albumin is not indicated as an intravenous nutrient. (1.5)
DOSAGE AND ADMINISTRATION
For intravenous use only.
- Adjust dose and rate of infusion based on the patient's clinical status. (2.1)
- Do not exceed 2 g of albumin per kg body weight for the daily dose. (2.1)
- Do not exceed 1 mL/min for patients with normal blood volume. (2.1)
- Do not dilute with Sterile Water for Injection. (2.2)
Indication Dose Hypovolemic Shock Infants and young children: 2.5 to 5 mL per kg body weight.
Older children and adults: initial dose 100 to 200 mL.
Repeat after 15 to 30 minutes if the response is not adequate.Hypoalbuminemia Calculate the body albumin compartment to be 80 to 100 mL per kg body weight. Do not exceed a daily dose of 2 g of albumin per kg of body weight. Burns The dosage should be determined according to the patient's condition and response to treatment after the first 24 hours. Hemolytic Disease of the Newborn 1 g per kg body weight prior to or during exchange transfusion.12 DOSAGE FORMS AND STRENGTHS
FLEXBUMIN 25% is a solution containing 25 g of albumin per each 100 mL. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions (including anaphylactic reactions) have been observed. If hypersensitivity reaction is suspected, discontinue use and implement appropriate standard medical treatment. (5.1)
- Under conditions where hypervolemia and/or hemodilution may occur, adjust the dose and rate of infusion to the patient's volume status. When administering large volumes, monitor hemodynamic parameters and ensure adequate substitution of other blood constituents are available (coagulation factors, platelets, and erythrocytes). Monitor electrolyte balance. (5.2)
- Closely monitor hemodynamic parameters after administration for evidence of cardiac or respiratory failure, renal failure or increasing intracranial pressure. (5.3)
- Monitor blood pressure in trauma patients and postoperative surgery patients in order to detect re-bleeding secondary to clot disruption. (5.4)
- Do not dilute with Sterile Water for Injection as this can cause hemolysis in recipients. (5.5)
This product is made from human plasma and may contain infectious agents e.g., viruses and, theoretically, the variant Creutzfeldt-Jakob disease agent. (5.6)
ADVERSE REACTIONS
The most serious adverse reactions are hypersensitivity reaction (including anaphylactic reaction) and pulmonary edema. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals U.S.A., Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Hypovolemia
1.2 Hypoalbuminemia
1.3 Cardiopulmonary Bypass Surgery
1.4 Hemolytic Disease of the Newborn (HDN)
1.5 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Hypervolemia/Hemodilution
5.3 Hemodynamics
5.4 Blood Pressure
5.5 Hemolysis
5.6 Transmission of Infectious Agents
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
FLEXBUMIN 25% [Albumin (Human)] is indicated for hypovolemia, hypoalbuminemia, cardiopulmonary bypass surgery, and hemolytic disease of the newborn (HDN).
1.1 Hypovolemia
FLEXBUMIN 25% [Albumin (Human)] is indicated for reversing hypovolemia. When hypovolemia is long standing and hypoalbuminemia exists accompanied by adequate hydration or edema, 25% albumin should be used.4,6
1.2 Hypoalbuminemia
FLEXBUMIN 25% is indicated for patients with hypoalbuminemia resulting from one or more of the following:5
- (1)
- Inadequate production (e.g., malnutrition, burns, major injury, infections)
- (2)
- Excessive catabolism (e.g., burns, major injury, pancreatitis)
- (3)
- Loss from the body (e.g., hemorrhage, excessive renal excretion, burn exudates)
- (4)
- Redistribution within the body (e.g., major surgery, various inflammatory conditions)
FLEXBUMIN 25% is indicated for patients with hypoalbuminemia accompanying severe injuries, infections or severe pancreatitis that cannot be quickly reversed and nutritional supplements fail to restore serum albumin levels.
Burns
After the first 24 hours, FLEXBUMIN 25% is indicated, in conjunction with appropriate crystalloid therapy, for the treatment of oncotic deficits following extensive burns and to replace the protein loss which accompanies any severe burn.4,6
1.3 Cardiopulmonary Bypass Surgery
Preoperative dilution of blood using albumin and crystalloid can be used in cardiopulmonary bypass surgery. FLEXBUMIN 25% is indicated as a component of the pump priming during cardiopulmonary bypass procedures.4,6,12
-
2 DOSAGE AND ADMINISTRATION
For intravenous use only.
2.1 Dose
The dose required depends on the patient's body weight, severity of injury/illness and on continuing fluid and protein losses. Adjust the concentration, dosage and infusion rate to the patient's individual requirements. Use adequacy of circulating blood volume, not plasma albumin levels, to determine the dose required. Refer to Table 1 for recommended doses.
Do not exceed 2 g of albumin per kg of body weight for the daily dose. Do not exceed 1 mL/min for patients with normal blood volume. More rapid administration can cause circulatory overload and pulmonary edema11 [see Warnings and Precautions (5.2)].
Table 1: Recommended Dose Indication Dose Hypovolemic Shock Infants and young children: 2.5 to 5 mL per kg body weight.
Older children and adults: initial dose 100 to 200 mL.
Repeat after 15 to 30 minutes if response is not adequate.Hypoalbuminemia Calculate the body albumin compartment to be 80 to 100 mL per kg body weight. Do not exceed a daily dose of 2 g of albumin per kg of body weight. Burns The dosage should be determined according to the patient's condition and response to treatment after the first 24 hours. Hemolytic disease in newborn 1 g per kilogram body weight prior to or during exchange transfusion.12 2.2 Administration
- Visually inspect parenteral drug product for particulate matter and discoloration prior to administration. FLEXBUMIN 25% is a transparent or slightly opalescent solution, which may have a greenish tint or may vary from a pale straw to an amber color. Do not use unless solution is clear of particulate matter or if the solution is turbid.
- Check the container for minute leaks prior to use by squeezing the bag firmly. If leaks are found, discard solution.
- Do not use the bag if the tip protector is damaged, detached or missing.
- Do not dilute with Sterile Water for Injection. Acceptable diluents include 0.9% Sodium Chloride or 5% Dextrose in Water [see Warnings and Precautions (5.5)].
- Do not mix or add with other medicinal products including blood and blood components, protein hydrolysates or solutions containing alcohol. Do not add supplementary medication.
- Administer within 4 hours after the container has been entered.
- Monitor hemodynamic parameters in patients receiving FLEXBUMIN 25% and check for the risk of hypervolemia and cardiovascular overload [see Warnings and Precautions (5.2)].
- Record the name and batch number of the product to maintain a link between the patient and the product.
- Discard unused portion.
CAUTION: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before the administration of fluid from the secondary container is complete.
- Suspend container from eyelet support.
- Remove plastic protector from outlet port at bottom of container.
- Attach administration set. Refer to complete directions accompanying the administration set.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- Patients with a history of hypersensitivity reaction to albumin preparations or to any of the excipients (N-acetyltryptophan and sodium caprylate). Reactions have included anaphylactic shock, anaphylactic reaction, or hypersensitivity/allergic reactions [see Warnings and Precautions (5.1), Adverse Reactions (6.2)].
- Patients with severe anemia or cardiac failure with normal or increased intravascular volume [see Warnings and Precautions (5.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions (including anaphylactic reactions) have been observed. Discontinue administration immediately if a hypersensitivity reaction (including anaphylactic type reactions) is suspected. In case of shock, implement standard medical treatment for shock.
5.2 Hypervolemia/Hemodilution
Under conditions where hypervolemia and/or hemodilution may occur, adjust dose and rate of infusion to the patient's volume status. Monitor coagulation and hematology parameters when comparatively large volumes are replaced. Ensure adequate substitution of other blood constituents (coagulation factors, platelets, and erythrocytes). Monitor electrolyte status to maintain the electrolyte balance.
Discontinue administration at the first clinical signs of cardiovascular overload (e.g., headache, dyspnea, jugular venous distention, rales and abnormal elevations in systemic or central venous blood pressure).
Conditions that pose increased risk of hypervolemia and/or hemodilution include but are not limited to:
- Heart failure
- Hypertension
- Esophageal varices
- Pulmonary edema
- Hemorrhagic diathesis
- Severe anemia
- Renal failure
5.3 Hemodynamics
Closely monitor hemodynamic parameters after administering FLEXBUMIN 25% for evidence of cardiac or respiratory failure, renal failure, or increasing intracranial pressure.
5.4 Blood Pressure
Monitor blood pressure in trauma patients and postoperative surgery patients resuscitated with FLEXBUMIN 25% in order to detect re-bleeding secondary to clot disruption.
5.5 Hemolysis
Do not dilute FLEXBUMIN 25% with Sterile Water for Injection as this can cause hemolysis in recipients. There exists a risk of potentially fatal hemolysis and acute renal failure from the use of Sterile Water for Injection as a diluent for Albumin (Human) in concentrations of 20% or higher [see Dosage and Administration (2.2)].
5.6 Transmission of Infectious Agents
FLEXBUMIN 25% is a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk of transmission would also be considered extremely remote. No cases of transmission of viral diseases, CJD or vCJD, have ever been identified for licensed albumin.
All infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Takeda Pharmaceuticals U.S.A., Inc. at 1-877-TAKEDA-7 (1-877-825-3327). The physician should discuss the risks and benefits of this product with the patient.
-
6 ADVERSE REACTIONS
The most serious adverse reactions are hypersensitivity reaction (including anaphylactic reaction) and pulmonary edema.
6.1 Clinical Trials Experience
No sponsor initiated clinical studies have been conducted with FLEXBUMIN 25%.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of FLEXBUMIN 25%. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions have been reported in the postapproval use of FLEXBUMIN 25%:
- Immune System Disorders: Anaphylactic shock, anaphylactic reaction, hypersensitivity/allergic reactions
- Nervous System Disorders: Headache, dysgeusia
- Cardiac Disorders: Myocardial infarction, atrial fibrillation, tachycardia
- Vascular Disorders: Hypotension, flushing
- Respiratory, Thoracic, and Mediastinal Disorders: Pulmonary edema, dyspnea
- Gastrointestinal Disorders: Vomiting, nausea
- Skin and Subcutaneous Tissue Disorders: Urticaria, rash, pruritus
- General Disorders and Administration Site Conditions: Pyrexia, chills
- 8 USE IN SPECIFIC POPULATIONS
-
10 OVERDOSAGE
Hypervolemia may occur if the dosage and rate of infusion are too high [see Warnings and Precautions (5.2)].
-
11 DESCRIPTION
FLEXBUMIN 25% is a sterile, nonpyrogenic preparation of albumin in a single dosage form for intravenous administration. Each 100 mL contains 25 g of albumin. It has been adjusted to physiological pH with sodium bicarbonate and/or sodium hydroxide and stabilized with N-acetyltryptophan (0.02M) and sodium caprylate (0.02M). The sodium content is 145 ± 15 mEq/L. FLEXBUMIN 25% contains no preservative and none of the coagulation factors found in fresh whole blood or plasma. FLEXBUMIN 25% is a transparent or slightly opalescent solution which may have a greenish tint or may vary from a pale straw to an amber color and is clear of particulate matter.
FLEXBUMIN 25% is manufactured from human plasma by the modified Cohn-Oncley cold ethanol fractionation process, which includes a series of cold-ethanol precipitation, centrifugation and/or filtration steps followed by pasteurization of the final product at 60 ± 0.5°C for 10 to 11 hours. This process accomplishes both purification of albumin and reduction of viruses.
In vitro studies demonstrate that the manufacturing process for FLEXBUMIN 25% provides for effective viral reduction. These viral reduction studies, summarized in Table 2, demonstrate viral clearance during the manufacturing process for FLEXBUMIN 25%.
These studies indicate that specific steps in the manufacturing of FLEXBUMIN 25% are capable of eliminating/inactivating a wide range of relevant and model viruses. Since the mechanism of virus elimination/inactivation by fractionation and by heating steps is different, the overall manufacturing process of FLEXBUMIN 25% is effective in reducing viral load.
Table 2: Summary of Viral Reduction Factor for Each Virus and Processing Step* Process Step Viral Reduction Factor (log10) Lipid Enveloped Non-Enveloped HIV-1 Flaviviridae PRV HAV Parvoviridae BVDV WNV MMV - *
- Human immunodeficiency virus, type 1 (HIV-1) both as a target virus and model for HIV-2 and other lipid-enveloped RNA viruses; bovine viral diarrhea virus (BVDV), a model for lipid-enveloped RNA viruses, such as hepatitis C virus (HCV); West Nile Virus (WNV), a target virus and model for other similar lipid-enveloped RNA viruses; pseudorabies virus (PRV), a model for other lipid-enveloped DNA viruses such as hepatitis B virus (HBV); mice minute virus (MMV), models for non-enveloped DNA viruses such as human parvovirus B1910; and hepatitis A virus (HAV), a target virus and a model for other non-enveloped RNA viruses.
- †
- Other Albumin fractionation process steps (processing of cryo-poor plasma to Fraction I + II + III/II + III supernatant and processing of Fraction V suspension to Cuno 90LP filtrate) showed virus reduction capacity in in vitro viral clearance studies. These process steps also contribute to the overall viral clearance effectiveness of the manufacturing process. However, since the mechanism of virus removal is similar to that of this particular process step, the viral inactivation data from other steps were not used in the calculation of the Mean Cumulative Reduction Factor.
- ‡
- n.d. = not determined.
- §
- Recent scientific data suggests that the actual human parvovirus B19 (B19V) is far more effectively inactivated by pasteurization than indicated by model virus data.10
Processing of Fraction I + II + III/II + III supernatant to Fraction IV4 Cuno 70C filtrate† >4.9 >4.8 >5.7 >5.5 >4.5 3.0 Pasteurization >7.8 >6.5 n.d.‡ >7.4 3.2 1.6§ Mean Cumulative Reduction Factor, log10 >12.7 >11.3 >5.7 >12.9 >7.7 4.6 The likelihood of the presence of viable hepatitis viruses has been minimized by testing the plasma at three stages: for the presence of hepatitis viruses, by fractionation steps with demonstrated virus removal capacity and by heating the product for 10 hours at 60°C. This procedure has been shown to be an effective method of inactivating hepatitis virus in albumin solutions even when those solutions were prepared from plasma known to be infective.1,2,3
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Albumin is responsible for 70 to 80% of the colloid osmotic pressure of normal plasma, thus making it useful in regulating the volume of circulating blood.4,5,6 Albumin is also a transport protein and binds naturally occurring, therapeutic and toxic materials in the circulation.5,6
12.2 Pharmacodynamics
FLEXBUMIN 25% is osmotically equivalent to approximately five times its volume of human plasma. When injected intravenously, 25% albumin will draw about 3.5 times its volume of additional fluid into the circulation within 15 minutes, except when the patient is markedly dehydrated. This extra fluid reduces hemoconcentration and blood viscosity. The degree and duration of volume expansion depends upon the initial blood volume. In patients with decreased blood volume, the effect of infused albumin can persist for many hours; however, in patients with normal blood volume, the duration will be shorter.7,8,9
12.3 Pharmacokinetics
Total body albumin is estimated to be 350 g for a 70 kg patient, with more than 60% located in the extravascular fluid compartment. The half-life of albumin is 15 to 20 days with a turnover of approximately 15 g per day.5
The minimum plasma albumin level necessary to prevent or reverse peripheral edema is unknown. It is recommended that plasma albumin levels be maintained at approximately 2.5 g/dL. This concentration provides a plasma oncotic pressure value of 20 mmHg.4
-
15 REFERENCES
- Cai K, Gierman T, Hotta J, et al: Ensuring the Biologic Safety of Plasma-Derived Therapeutic Proteins. Biodrugs 2005; 19 (2): 79-96.
- Gerety R, Aronson D: Plasma derivatives and viral hepatitis. Transfusion 1982; 22 (5): 347-351.
- Burnouf T, Padilla A: Current strategies to prevent transmission of prions by human plasma derivatives. Transfusion Clinique et Biologique 2006; 13: 320-328.
- Tullis J: Albumin 1. Background and use Albumin 2. Guidelines for clinical use. JAMA 1977; 237 (4): 355-360; 460-463.
- Peters T Jr: Serum albumin. The Plasma Proteins, 2nd Edition, Vol 1. Putnam FW (ed). New York, Academic Press, 1975, pp 133-181.
- Finlayson J: Albumin products. Seminars in Thrombosis and Hemostasis 1980; 6 (2): 85-120.
- Haynes G, Navickis R, Wilkes M: Albumin administration – what is the evidence of clinical benefit? A systematic review of randomized controlled trials. European Journal of Anesthesiology 2003; 20: 771-793.
- Mendez C, McClain C, Marsano L, et al: Albumin Therapy in Clinical Practice. Nutrition in Clinical Practice 2005; 20:314-320.
- Quinlan G, Martin G, Evans T: Albumin: Biochemical Properties and Therapeutic Potential. Hepatology 2005; 41 (6): 1211-1219.
- Blümel J, Schmidt I, Willkommen H, et al: Inactivation of parvovirus B19 during pasteurization of human serum albumin. Transfusion 2002; 42:1011-1018.
- Grocott M, Mythen M, Gan T: Perioperative Fluid Management and Clinical Outcomes in Adults. Anesth Analg. 2005; 100: 1093-1106.
- Tsao Y, Yu V: Albumin in management of neonatal hyperbilirubinaemia. Archives of Disease in Childhood 1972; 47: 250.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
FLEXBUMIN 25% is supplied in a single-dose plastic container:
Plastic Container NDC Number Carton NDC Number Grams Protein and Fill Size NDC 0944-0493-03
(quantity of 1)NDC 0944-0493-01
(quantity of 12)12.5 grams in a 50 mL plastic container NDC 0944-0493-04
(quantity of 1)NDC 0944-0493-02
(quantity of 6)25 grams in a 100 mL plastic container -
17 PATIENT COUNSELING INFORMATION
- Inform patients of the early signs of hypersensitivity reactions, including hives, generalized urticaria, chest tightness, dyspnea, wheezing, faintness, hypotension, and anaphylaxis [see Warnings and Precautions (5.1)].
- Inform patients that FLEXBUMIN 25% is made from human plasma and may contain infectious agents that can cause disease (e.g., viruses and, theoretically, the CJD agent). Explain that the risk of FLEXBUMIN 25% transmitting an infectious agent has been reduced by screening the plasma donors, by testing the donated plasma for certain virus infections, and by a process demonstrated to inactivate and/or remove certain viruses during manufacturing. Symptoms of a possible virus infection include headache, fever, nausea, vomiting, weakness, malaise, diarrhea, or, in the case of hepatitis, jaundice [see Warnings and Precautions (5.6)].
- SPL UNCLASSIFIED SECTION
-
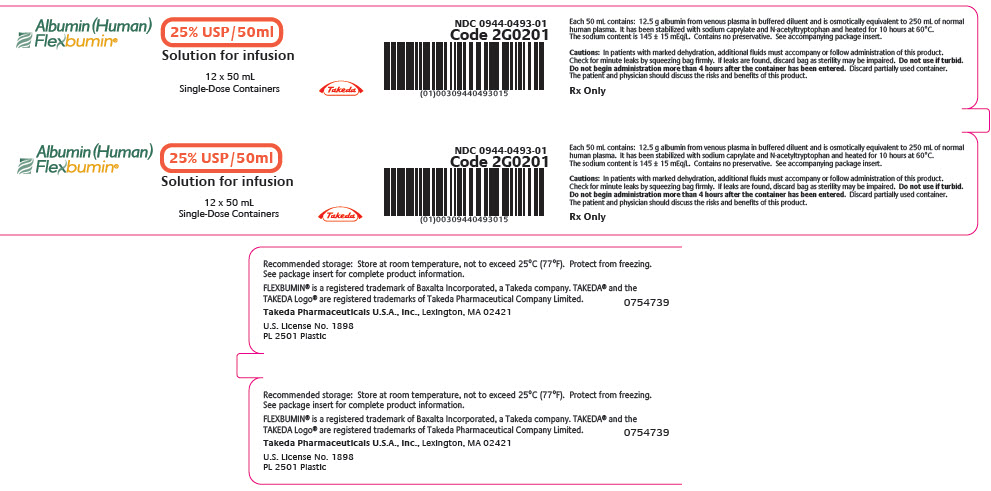
PRINCIPAL DISPLAY PANEL - 50 mL Bag Label
Albumin (Human)
Flexbumin®
25% USP/50ml
Solution for infusionTakeda
Single-Dose Container
50 mL
NDC 0944-0493-03
Code 2G0201Each 50 mL contains: 12.5 g albumin from venous plasma in buffered diluent
and is osmotically equivalent to 250 mL of normal human plasma. It has been
stabilized with sodium caprylate and N-acetyltryptophan and heated for 10 hours
at 60°C. The sodium content is 145 ± 15 mEq/L. Contains no preservative. See
accompanying package insert.Cautions: In patients with marked dehydration, additional fluids must accompany
or follow administration of this product. Check for minute leaks by squeezing bag
firmly. If leaks are found, discard bag as sterility may be impaired. Do not use if
turbid. Do not begin administration more than 4 hours after the container has
been entered. Discard partially used container. The patient and physician should
discuss the risks and benefits of this product.Rx Only
Recommended storage: Store at room temperature, not to exceed 25°C (77°F).
Protect from freezing.FLEXBUMIN® is a registered trademark of Baxalta Incorporated, a Takeda company.
TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical
Company Limited.Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898PL 2501 Plastic
0754738
- PRINCIPAL DISPLAY PANEL - 50 mL Carton Label
-
PRINCIPAL DISPLAY PANEL - 100 mL Bag Label
Albumin (Human)
Flexbumin®
25% USP/100ml
Solution for infusionTakeda
Single-Dose Container
100 mL
NDC 0944-0493-04
Code 2G0012Each 100 mL contains: 25 g albumin from venous plasma in
buffered diluent and is osmotically equivalent to 500 mL of normal
human plasma. It has been stabilized with sodium caprylate
and N-acetyltryptophan and heated for 10 hours at 60°C. The
sodium content is 145 ± 15 mEq/L. Contains no preservative. See
accompanying package insert.Cautions: In patients with marked dehydration, additional fluids must
accompany or follow administration of this product. Check for minute
leaks by squeezing bag firmly. If leaks are found, discard bag as sterility
may be impaired. Do not use if turbid. Do not begin administration
more than 4 hours after the container has been entered. Discard
partially used container. The patient and physician should discuss the
risks and benefits of this product.Rx Only
Recommended storage: Store at room temperature, not to exceed 25°C
(77°F). Protect from freezing.FLEXBUMIN® is a registered trademark of Baxalta Incorporated, a
Takeda company. TAKEDA® and the TAKEDA Logo® are registered
trademarks of Takeda Pharmaceutical Company Limited.Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898PL 2501 Plastic
0754742
- PRINCIPAL DISPLAY PANEL - 100 mL Carton Label
-
INGREDIENTS AND APPEARANCE
FLEXBUMIN
albumin human solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0944-0493 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) (ALBUMIN HUMAN - UNII:ZIF514RVZR) ALBUMIN HUMAN 0.25 g in 1 mL Inactive Ingredients Ingredient Name Strength N-ACETYL-DL-TRYPTOPHAN SODIUM (UNII: 3EN9H0M2FX) SODIUM CAPRYLATE (UNII: 9XTM81VK2B) SODIUM CATION (UNII: LYR4M0NH37) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-0493-01 12 in 1 CARTON 1 NDC:0944-0493-03 50 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC:0944-0493-02 6 in 1 CARTON 2 NDC:0944-0493-04 100 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101452 08/09/2002 Labeler - Takeda Pharmaceuticals America, Inc. (039997266) Establishment Name Address ID/FEI Business Operations BAXALTA INCORPORATED 085206634 MANUFACTURE(0944-0493) Establishment Name Address ID/FEI Business Operations Baxalta US Inc. 105875392 MANUFACTURE(0944-0493)