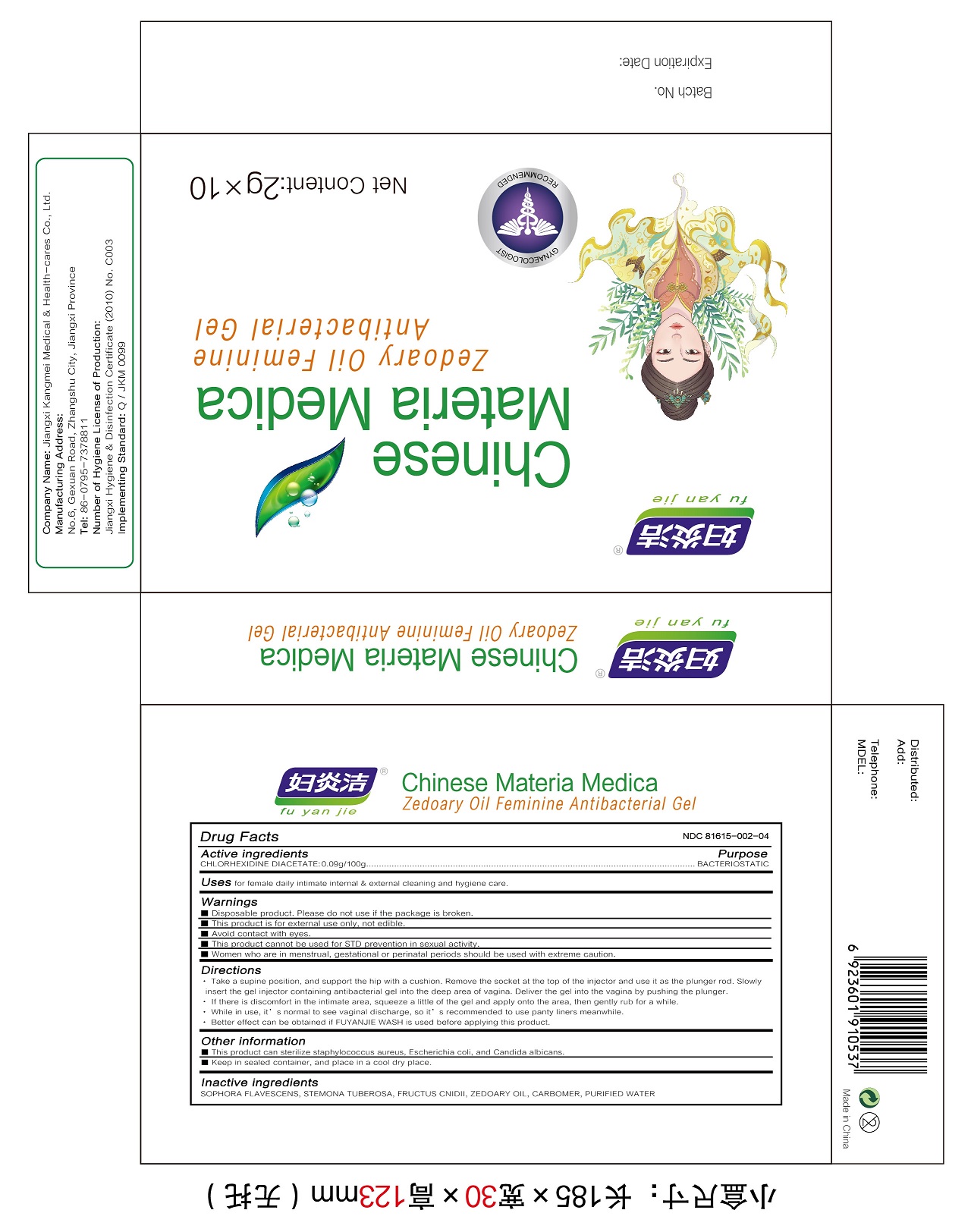
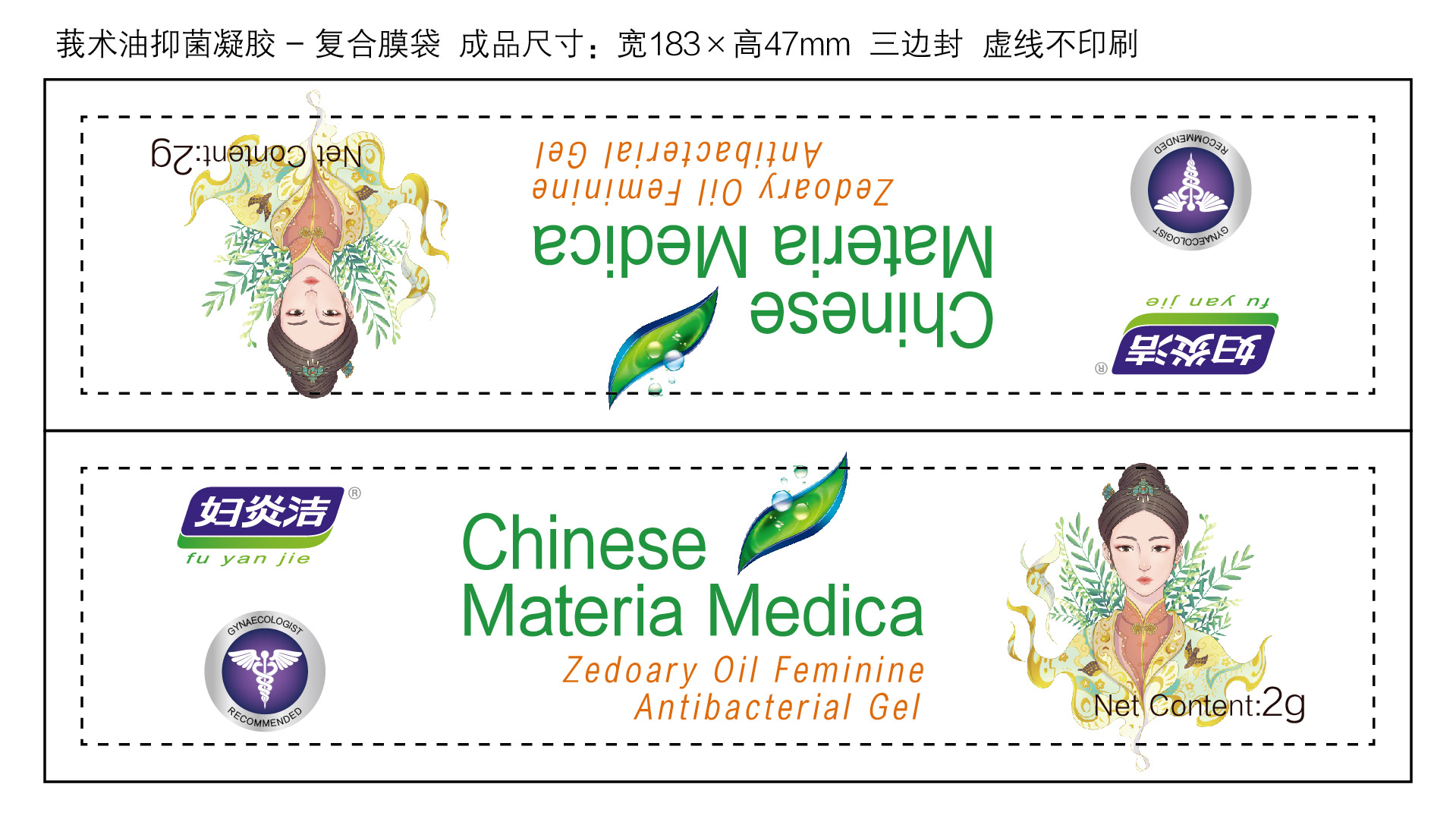
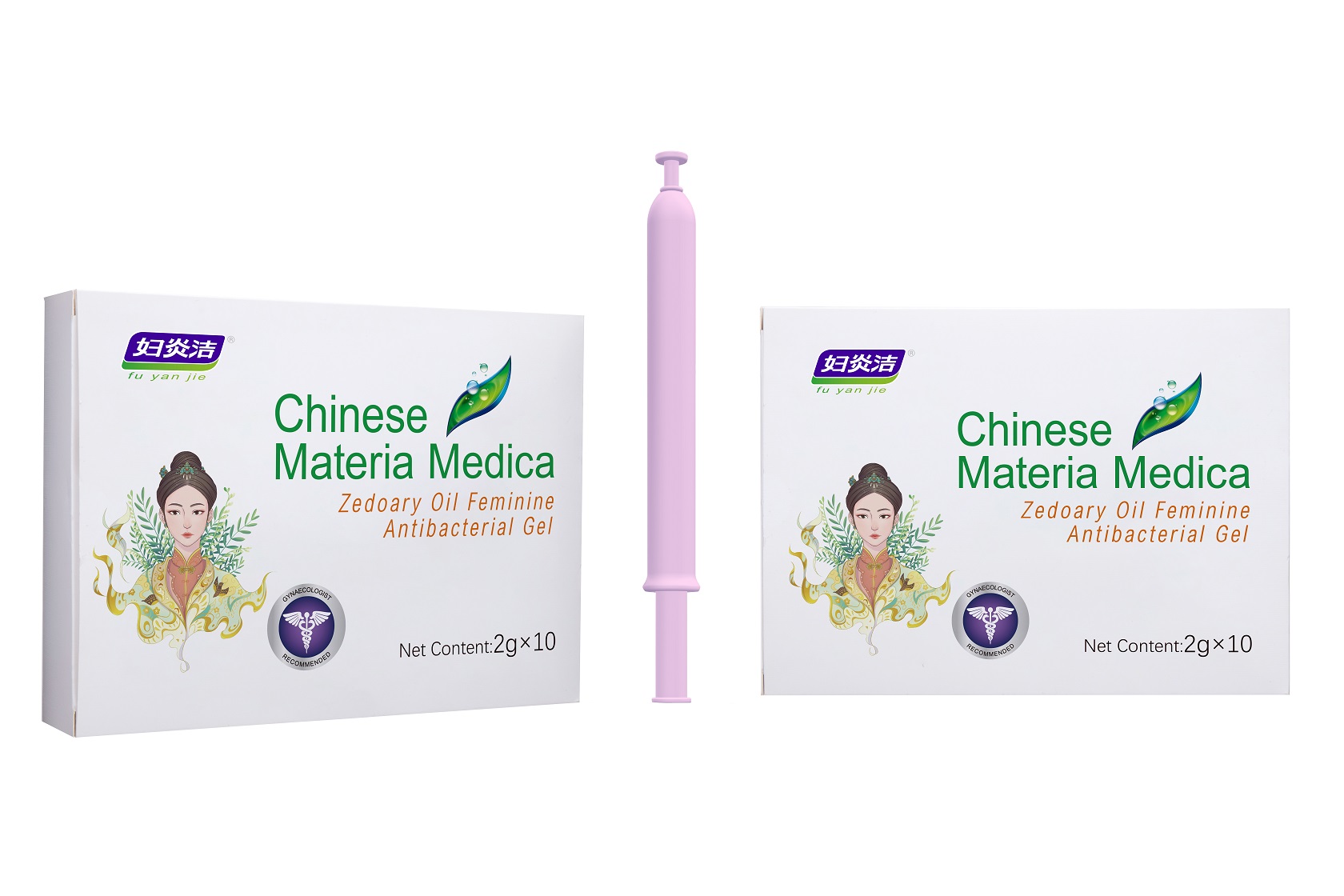
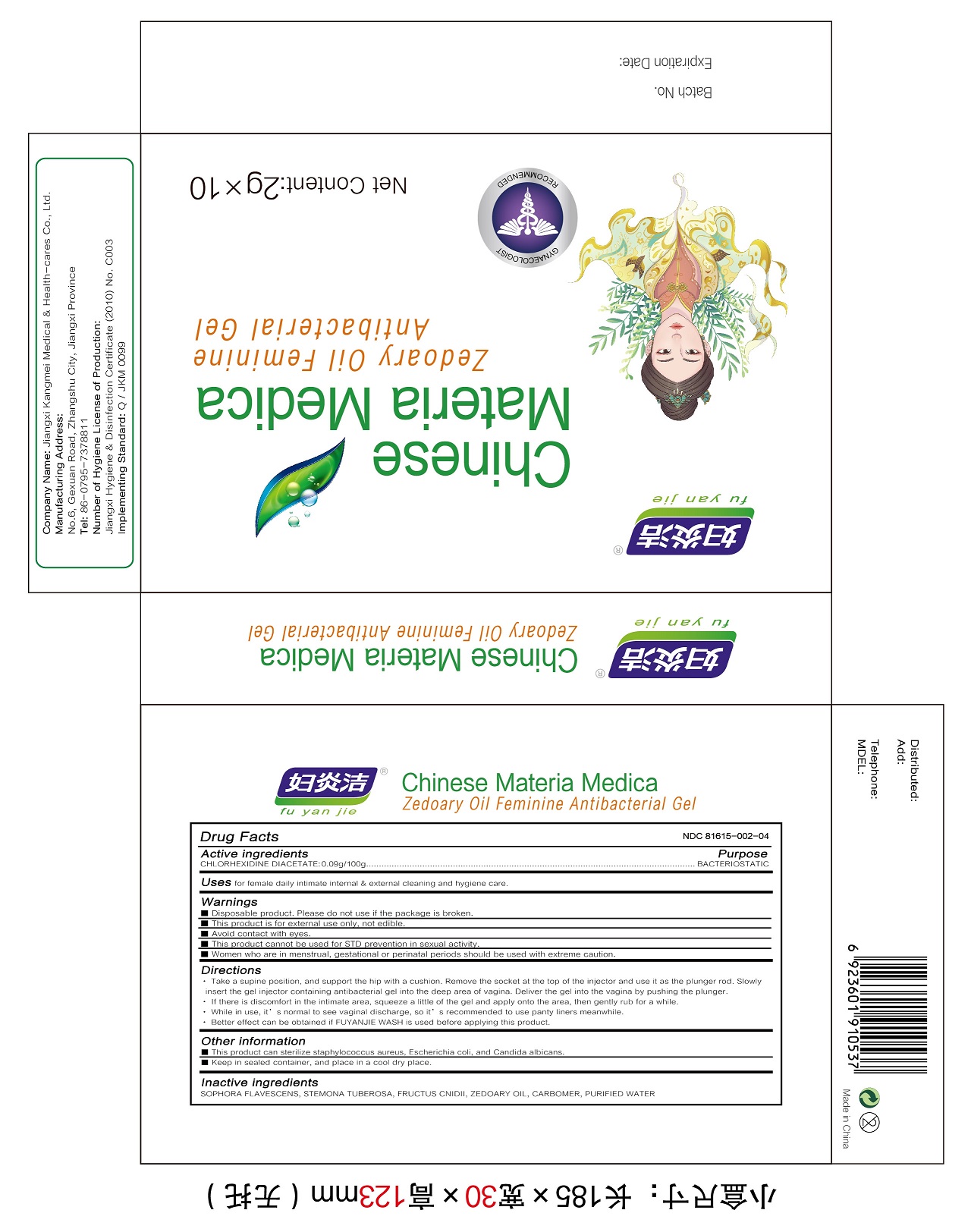
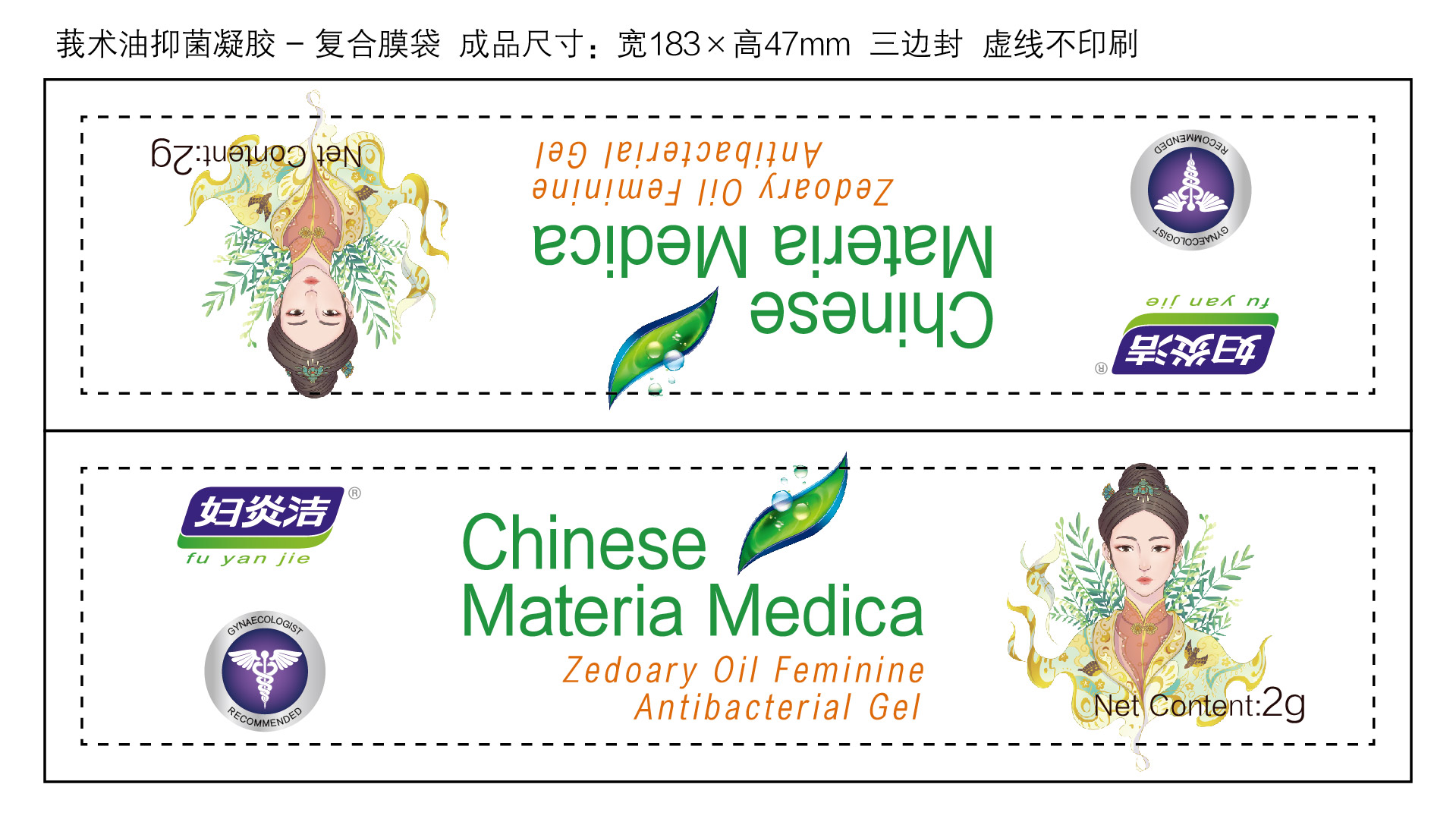
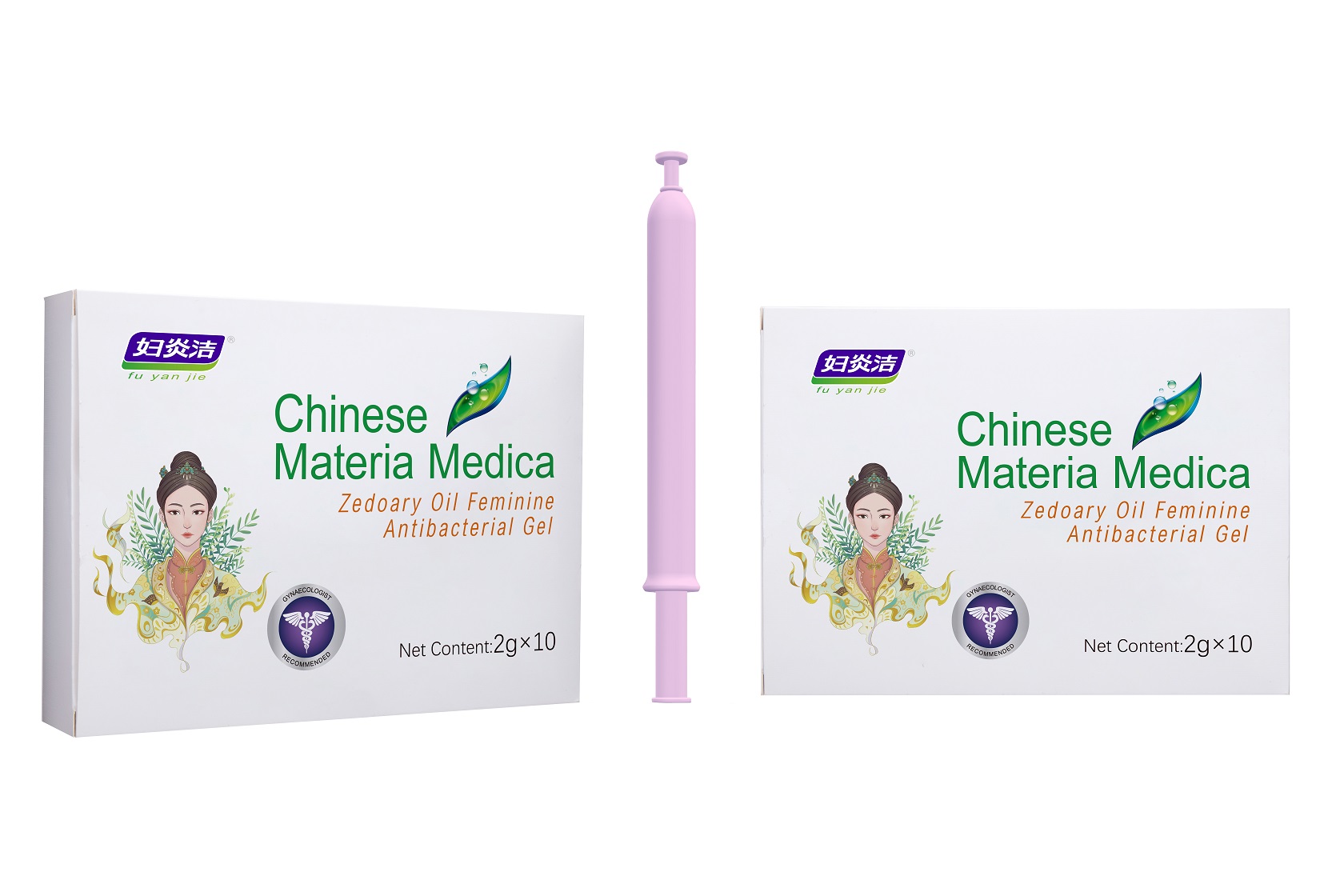
Label: ZEDOARY OILS FEMININE ANTIBACTERIAL GELS- chlorhexidine diacetate gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 81615-002-01, 81615-002-02, 81615-002-03, 81615-002-04, view more81615-002-05, 81615-002-06 - Packager: Jiangxi Renhetang pharmaceutical chain Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 20, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient(s)
- Purpose
- Use
-
Warnings
Disposable product. Please do not use if the package is broken.
This product is for external use only, not edible.
Avoid contact with eyes
This product cannot be used for STD prevention in sexual activity.
Women who are in menstrual, gestational or perinatal periods should be used with extreme caution.
-
WHEN USING
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. - STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
1.Take a supine position, and support the hip with a cushion. Remove the socket at the top of the injector and use it as the plunger rod. Slowly insert the gel injector containing antibacterial gel into the deep area of vagina. Deliver the gel into the vagina by pushing the plunger.
2.If there is discomfort in the intimate area, squeeze a little of the gel and apply onto the area, then gently rub for a while.
3.While in use, it’s normal to see vaginal discharge, so it’s recommended to use panty liners meanwhile.
4.Better effect can be obtained if FUYANJIE WASH is used before applying this product. - Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ZEDOARY OILS FEMININE ANTIBACTERIAL GELS
chlorhexidine diacetate gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81615-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE ACETATE (UNII: 5908ZUF22Y) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE ACETATE 0.09 g in 100 g Inactive Ingredients Ingredient Name Strength ZEDOARY OIL (UNII: QPP5U30FSD) OSTHOL (UNII: XH1TI1759C) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) STEMONA TUBEROSA ROOT (UNII: 7S9328671Z) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81615-002-02 7 in 1 BOX 03/16/2021 1 NDC:81615-002-01 5 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:81615-002-04 10 in 1 BOX 03/16/2021 2 NDC:81615-002-03 2 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:81615-002-06 10 in 1 BOX 03/16/2021 3 NDC:81615-002-05 3 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/16/2021 Labeler - Jiangxi Renhetang pharmaceutical chain Co., Ltd. (410551226) Establishment Name Address ID/FEI Business Operations Jiangxi Kangmei medical and Health Products Co., Ltd 528118770 manufacture(81615-002)