Label: SOOTH-A-STING- benzocaine swab
- NDC Code(s): 50332-0050-0, 50332-0050-4
- Packager: HART Health
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 18, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
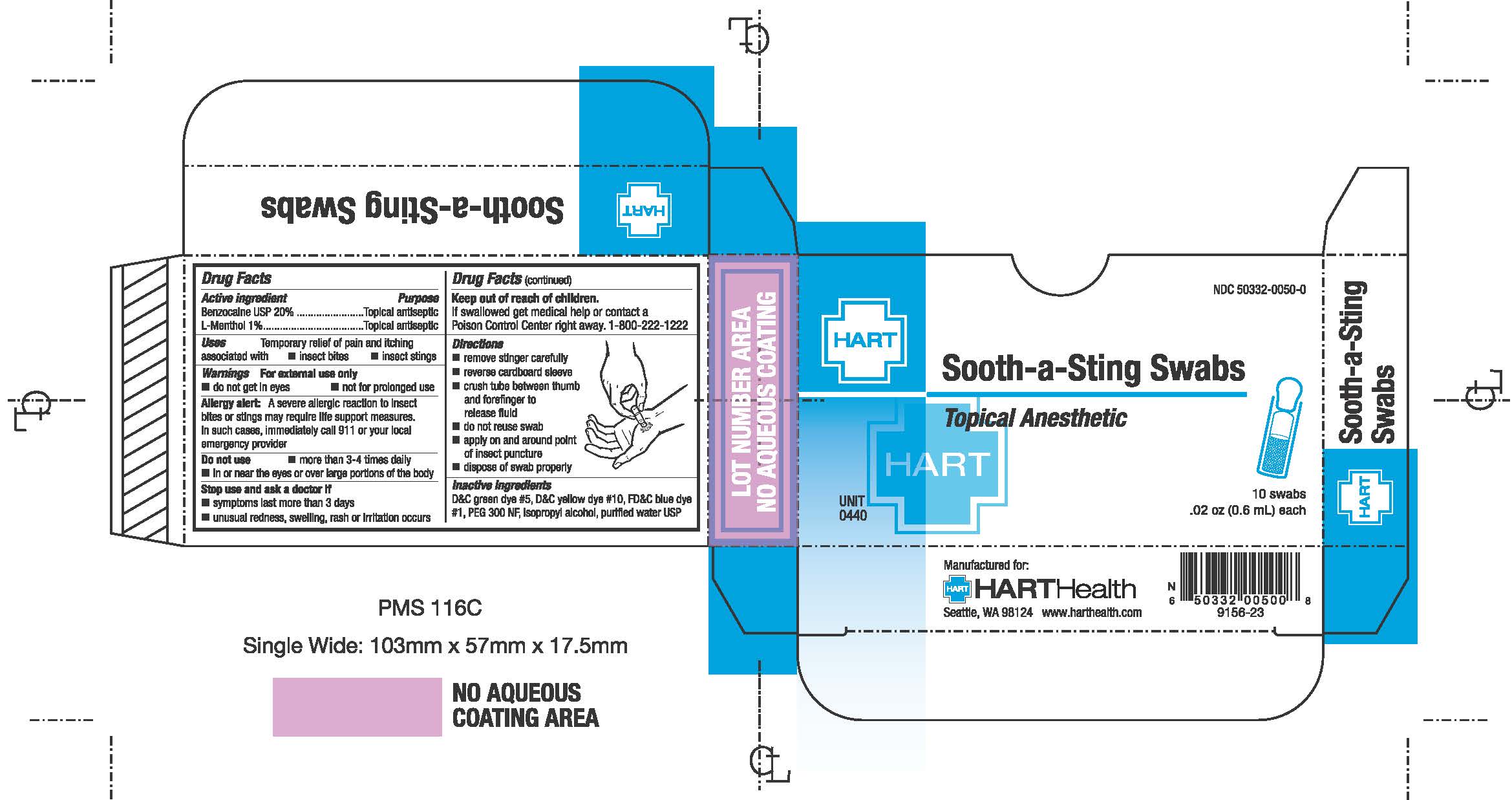
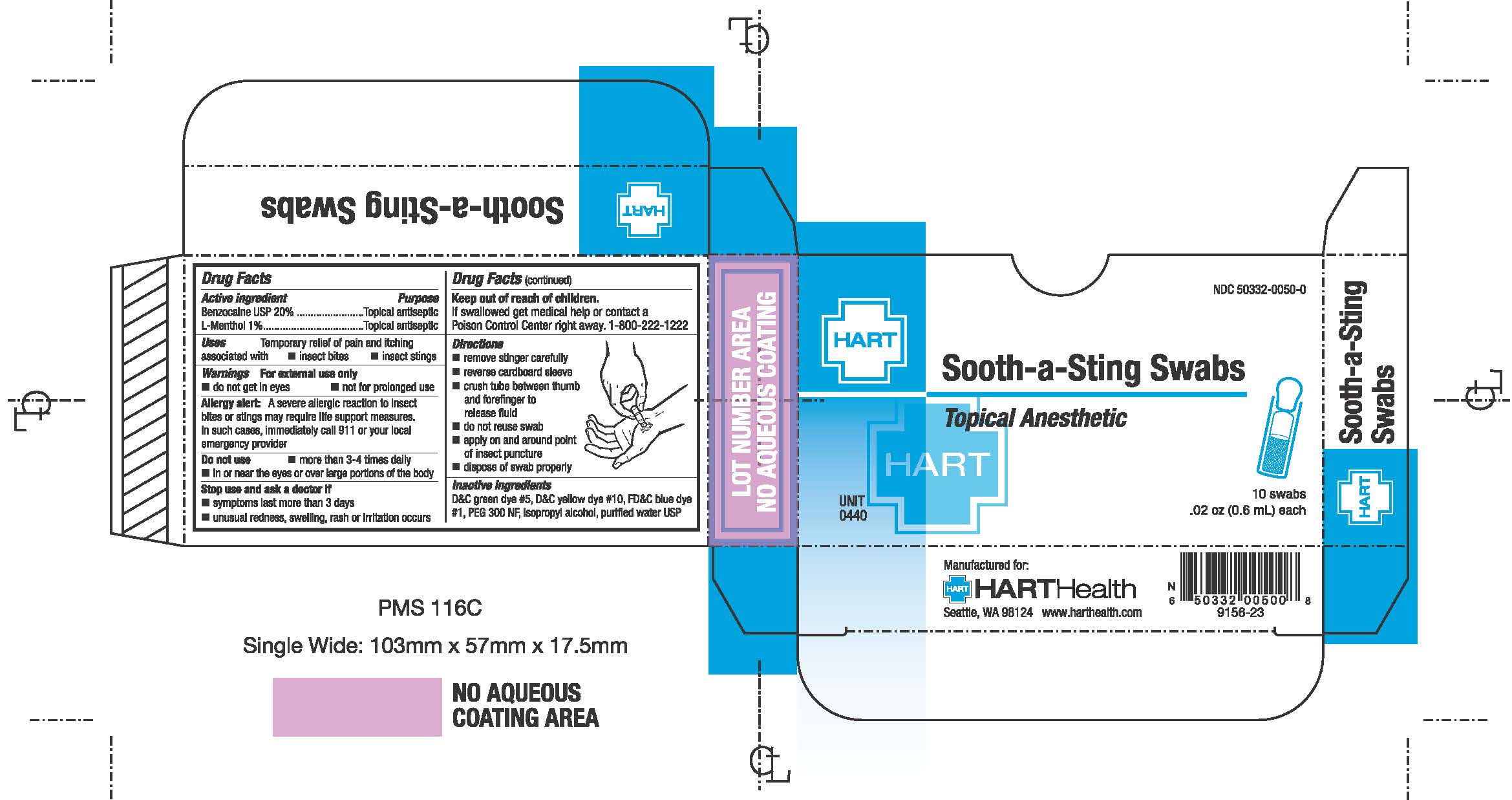
- Active Ingredients
- Purpose
- Uses
- Warnings
- Do not use
- Stop use and ask a doctor if
- Keep out of reach of children
- Directions
- Inactive Ingredient
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SOOTH-A-STING
benzocaine swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50332-0050 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 0.12 g in 0.6 mL MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM .006 g in 0.6 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) WATER (UNII: 059QF0KO0R) D&C GREEN NO. 5 (UNII: 8J6RDU8L9X) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) POLYETHYLENE GLYCOL 300 (UNII: 5655G9Y8AQ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50332-0050-0 10 in 1 BOX 01/01/2000 1 .6 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC:50332-0050-4 100 in 1 BAG 01/01/2000 2 .6 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M 01/01/2000 Labeler - HART Health (069560969) Registrant - HART Health (069560969) Establishment Name Address ID/FEI Business Operations JAMES ALEXANDER CORPORATION 040756421 manufacture(50332-0050)