Label: ANTI-DIARRHEAL- loperamide hydrochloride capsule, liquid filled
- NDC Code(s): 70000-0461-1
- Packager: CARDINAL HEALTH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each capsule)
- Purpose
- Use
- Warnings
- Do not use
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
CardinalHealth TM
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
www.myleader.com 1-800-200-6313
Essential to Care™ since 1979
All LEADER TM Brand Products Have A 100% Money Back Guarantee
Return to place of purchase if not satisfied.
†This product is not manufactured or distributed by Kenvue Inc., owner of the registered trademark Imodium ® A-D.
© 2018 Cardinal Health. All Rights Reserved.
CARDINAL HEALTH, the Cardinal Health LOGO,
ESSENTIAL TO CARE, LEADER, and the LEADER LOGO
are trademarks or registered trademarks of
Cardinal Health. All other marks are the property
of their respective owners.
THIS PRODUCT IS PACKAGED IN A CHILD-RESISTANT AND TAMPER
EVIDENT PACKAGE. USE ONLY IF BLISTERS ARE INTACT.
KEEP OUTER CARTON FOR COMPLETE
WARNINGS AND PRODUCT INFORMATION.
Manufactured for:
BIONPHARMA
Princeton, NJ 08540
L0000786
Lot#
Expiration Date
R0224
-
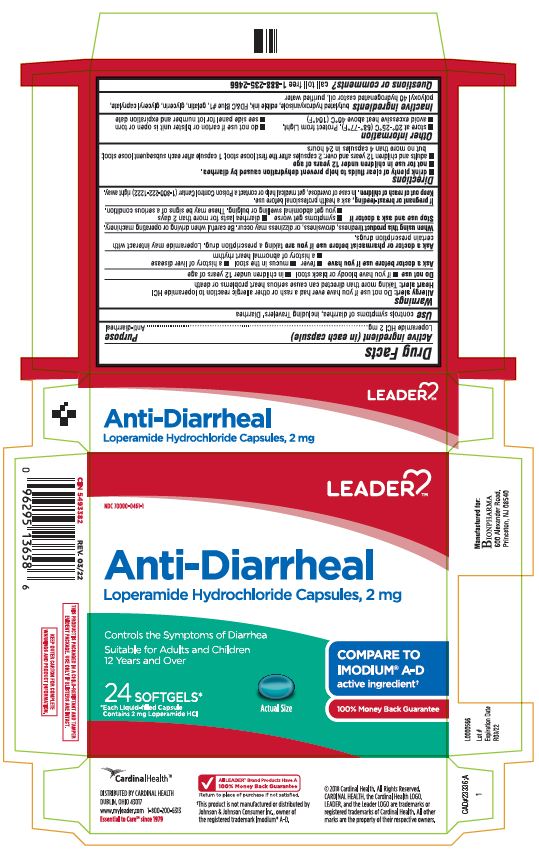
Principal Display Panel
LEADER TM
NDC 70000-0461-1
Anti-Diarrheal
Loperamide Hydrochloride Capsules, 2 mg
Controls the Symptoms of Diarrhea
Suitable for Adults and Children
12 years and over
24 SOFTGELS*
*Each Liquid-Filled Capsule
Contains 2 mg Loperamide HCl
Actual Size
COMPARE TO
IMODIUM ® A-D
active ingredient †
100 % Money Back Guarantee
-
INGREDIENTS AND APPEARANCE
ANTI-DIARRHEAL
loperamide hydrochloride capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70000-0461 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOPERAMIDE HYDROCHLORIDE (UNII: 77TI35393C) (LOPERAMIDE - UNII:6X9OC3H4II) LOPERAMIDE HYDROCHLORIDE 2 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) WATER (UNII: 059QF0KO0R) Product Characteristics Color blue Score no score Shape CAPSULE Size 10mm Flavor Imprint Code LP2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70000-0461-1 2 in 1 CARTON 01/21/2019 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021855 01/21/2019 Labeler - CARDINAL HEALTH (063997360) Registrant - Bionpharma Inc. (079637826) Establishment Name Address ID/FEI Business Operations Patheon Softgels Inc. 002193829 manufacture(70000-0461)

