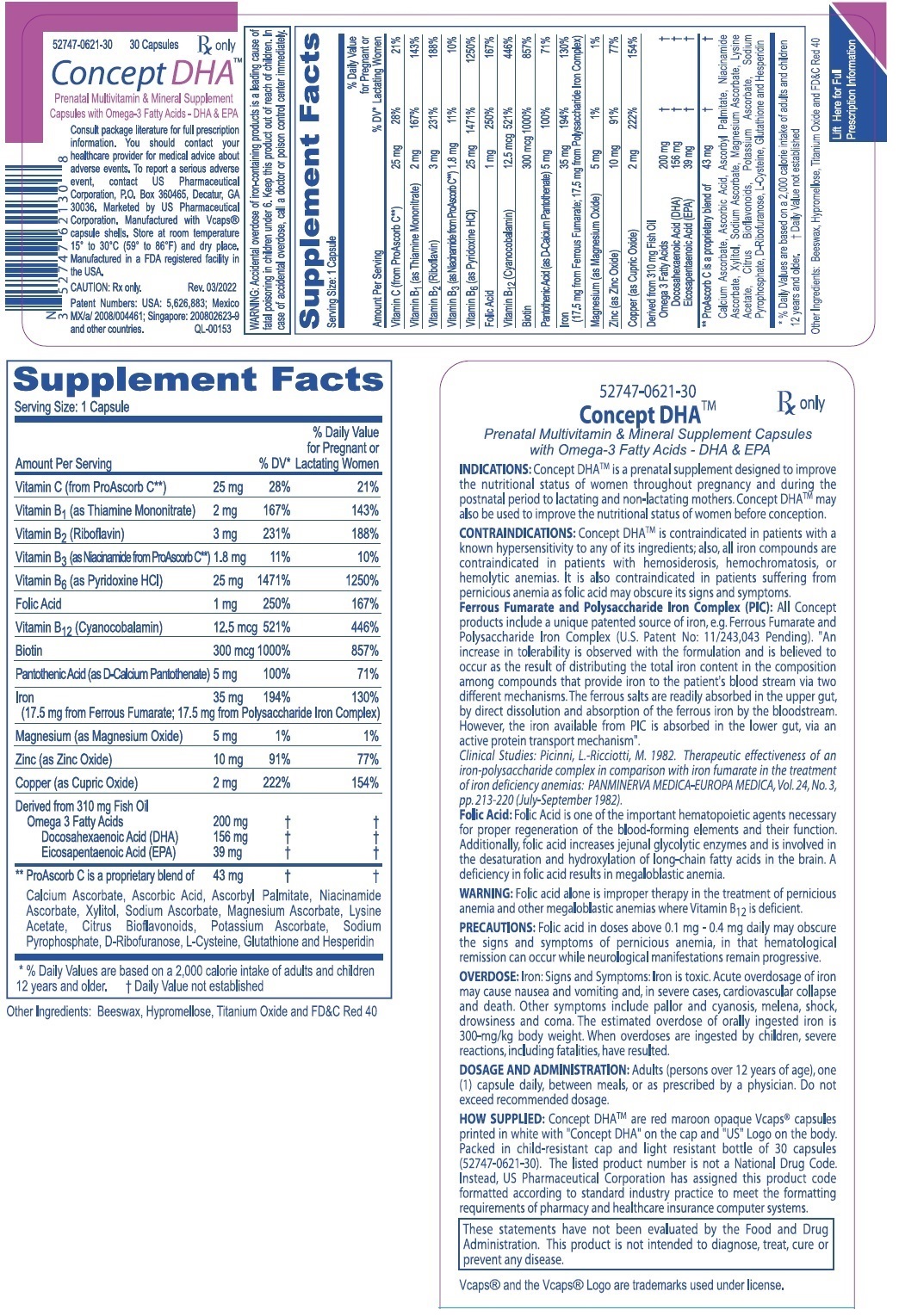
Label: CONCEPT DHA- vitamin- mineral omega-3 supplement capsule, liquid filled
- NDC Code(s): 52747-621-30
- Packager: U.S. Pharmaceutical Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 8, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
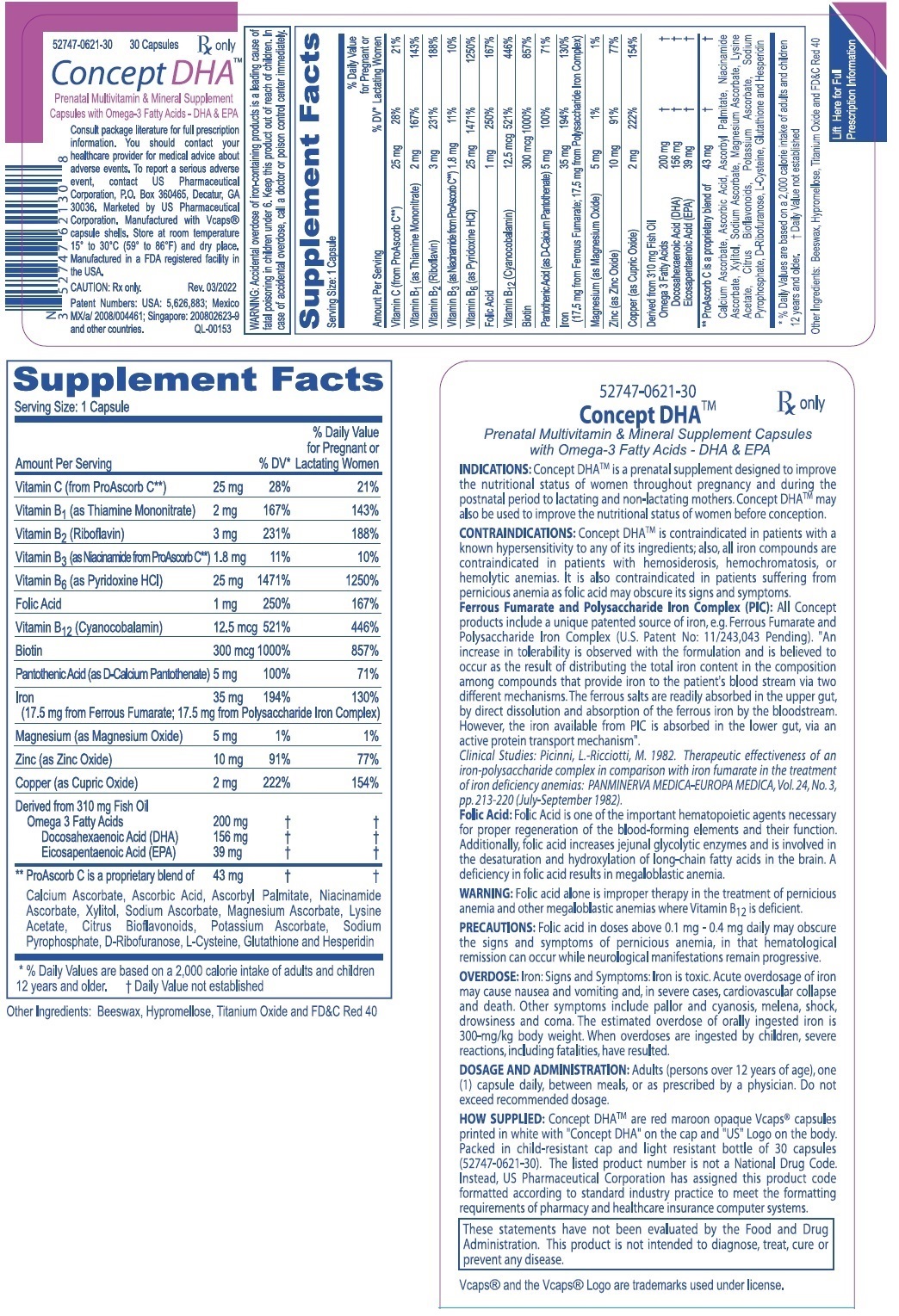
DESCRIPTION: Each capsule contains: Ferrous Fumarate (Elemental Iron) . . . . . . . . . . 17.5 mg PolysaccharideIronComplex(ElementalIron).. 17.5mg
(Equivalent to about 35 mg of elemental iron) Vitamin C (from ProAscorb C‡) . . . . . . . . . . . . 25 mg FolicAcid.............................. 1mg ThiamineMononitrate(B1) ................ 2mg Riboflavin(B2) .......................... 3mg Niacin (B3, from ProAscorb C‡) . . . . . . . . . . . . 1.8 mg d-CalciumPantothenate(B5)................ 5mg PyridoxineHCI(B6) .................... 25mg Biotin (B7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . 300 mcg Cyanocobalamin(B12) ................ 12.5mcg Copper(asCopperSulfate).................. 2mg
Magnesium(asMagnesiumSulfate) . . . . . . . . Zinc(asZincSulfate).................... Omega-3 Fatty Acids . . . . . . . . . ...........
(Derived from 310 mg Fish Oil) Docosahexaenoic Acid (DHA) . ........... Eicosapentaenoic Acid (EPA) . ........... . 5mg 10mg 200 mg156 mg 39 mg
Clinical Studies: Because Ferrous Fumarate is an organic complex, it contains no free ions, either ferric or ferrous. Polysaccharide Iron Complex is clinically non-toxic. Prior studies in rats demonstrated that Polysaccharide Iron Complex (PIC), administered as a single oral dose to Sprague Dawley rats did not produce evidence of toxicity at a dosage level of 5000 mg Iron/kg: (An Acute Oral Toxicity Study in Rats with Polysaccharide-Iron Complex. T.N.Merriman, M. Aikman and R.E. Rush, Springborn Laboratories, Inc. Spencerville, Ohio Study No. 3340.1 March - April 1994). Other clinical studies had demonstrated that Polysaccharide Iron gives a good hematopoietic response with an almost complete absence of the side effects usually associated with oral iron therapy. Picinni and Ricciotti suggested in 1982, that "the therapeutic effectiveness of Polysaccharide Iron Complex when compared with iron fumarate in the treatment of iron deficiency anemia, appears to be as active as the iron fumarate and as well tolerated, however, it exerted a greater influence on the level of hemoglobin and on the number of red cells..." and that, "it has been exceptionally well tolerated by all patients" (Picinni, L.-Ricciotti, M. 1982. Therapeutic effectiveness of an iron-polysaccharide complex in comparison with iron fumarate in the treatment of iron deficiency anemias): PANMINERVA MEDICA-EUROPA MEDICA, Vol. 24, No. 3, pp. 213-220 (July - September 1982). As mentioned above, the patented source of iron used in Concept DHATM (Ferrous Fumarate and Polysaccharide Iron Complex) provides a high level of elemental iron with a low incidence of gastric distress.
CONCLUSION: Based on the results of this study, the oral combination of Ferrous Fumarate and Polysaccharide Iron Complex was better tolerated and safer than the oral administration of Ferrous Fumarate alone. The conclusion of this research stated, that the addition of PIC to Ferrous Fumarate surprisingly allows the same concentration of Ferrous Fumarate to be better tolerated than the Ferrous Fumarate alone.INDICATIONS: Concept DHATM is a prescription prenatal vitamin-mineral preparation containing omega-3 fatty acid supplements designed to supply nutritional supplementation for women throughout pregnancy and during the postnatal period to lactating and non-lactating mothers. Concept DHATM may also be used to improve the nutritional status of women before conception.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately. WARNING: Ingestion of more than 3 grams of omega-3 fatty acids from fish oils per day may have potential antithrombotic effects, including an increased bleeding time and INR (international normalized ratio). DHA should be avoided in patients with inherited or acquired bleeding diatheses, including those taking anticoagulants.
WARNING: Folic acid alone is improper therapy in the treatment for pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient.
PRECAUTIONS: General: Folic acid in doses above 0.1 mg - 0.4 mg daily may obscure pernicious anemia, in that hematological remission can occur while neurological manifestations remain progressive.
DOSAGE AND ADMINISTRATION: Adults (persons over 12 years of age), one (1) capsule daily, between meals, or as prescribed by a physician. Do not exceed recommended dosage. Do not administer to children under the age of 12.
HOW SUPPLIED: Concept DHATM are red maroon opaque Vcaps® capsules printed in white with "Concept DHA" on the cap "US" logo on the body. Packed in child-resistant cap and light resistant bottle of 30 capsules (52747-0621-30). The listed product number is not a National Drug Code. Instead, US Pharmaceutical Corporation has assigned this product code formatted according to standard industry practice to meet the formatting requirements of pharmacy and healthcare insurance computer systems.
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease. Vcaps® and the Vcaps® Logo are trademarks used under license.
- Packaging
-
INGREDIENTS AND APPEARANCE
CONCEPT DHA
vitamin- mineral omega-3 supplement capsule, liquid filledProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:52747-621 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 17.5 mg IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 17.5 mg ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 25 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 2 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3 mg NIACIN (UNII: 2679MF687A) (NIACIN - UNII:2679MF687A) NIACIN 1.8 mg CALCIUM PANTOTHENATE (UNII: 568ET80C3D) (PANTOTHENIC ACID - UNII:19F5HK2737) PANTOTHENIC ACID 5 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 25 mg BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 300 ug CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 12.5 ug CUPRIC SULFATE (UNII: LRX7AJ16DT) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 2 mg MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM SULFATE, UNSPECIFIED FORM 5 mg ZINC SULFATE, UNSPECIFIED FORM (UNII: 89DS0H96TB) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 10 mg OMEGA-3-ACID ETHYL ESTERS (UNII: D87YGH4Z0Q) (OMEGA-3 FATTY ACIDS - UNII:71M78END5S) OMEGA-3-ACID ETHYL ESTERS 200 mg Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color pink (pink) Score score with uneven pieces Shape CAPSULE Size 22mm Flavor Imprint Code Concept;DHA;US Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52747-621-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/24/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/24/2009 Labeler - U.S. Pharmaceutical Corporation (079467662)