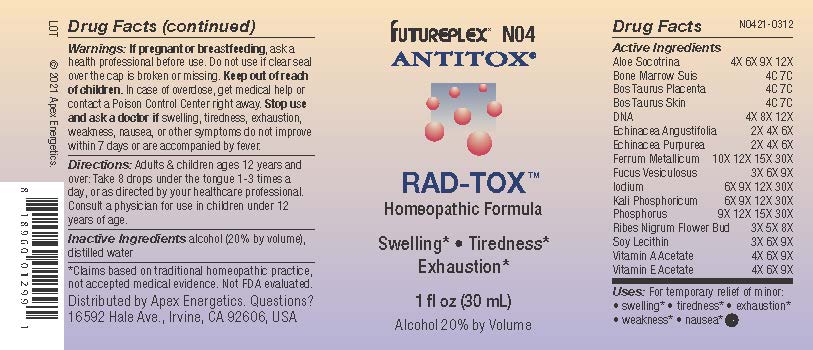
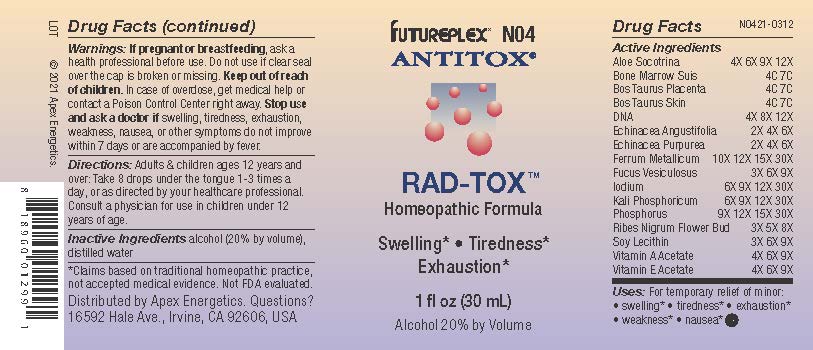
Label: N04 RAD-TOX- aloe socotrina, bone marrow suis, bos taurus placenta, bos taurus skin, dna, echinacea angustifolia, echinacea purpurea, ferrum metallicum, fucus vesiculosus, iodium, kali phosphoricum, phosphorus, ribes nigrum flower bud, soy lecithin, vitamin a acetate, vitamin e acetate solution/ drops
- NDC Code(s): 63479-1404-1
- Packager: Apex Energetics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients
Aloe Socotrina
4X 6X 9X 12X
Bone Marrow Suis
4C 7C
Bos Taurus Placenta
4C 7C
Bos Taurus Skin
4C 7C
Dna
4X 8X 12X
Echinacea Angustifolia
2X 4X 6X
Echinacea Purpurea
2X 4X 6X
Ferrum Metallicum
10X 12X 15X 30X
Fucus Vesiculosus
3X 6X 9X
Iodium
6X 9X 12X 30X
Kali Phosphoricum
6X 9X 12X 30X
Phosphorus
9X 12X 15X 30X
Ribes Nigrum Flower Bud
3X 5X 8X
Soy Lecithin
3X 6X 9X
Vitamin A Acetate
4X 6X 9X
Vitamin E Acetate
4X 6X 9X
- INDICATIONS & USAGE
- Warnings:
- Directions:
- Inactive Ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
N04 RAD-TOX
aloe socotrina, bone marrow suis, bos taurus placenta, bos taurus skin, dna, echinacea angustifolia, echinacea purpurea, ferrum metallicum, fucus vesiculosus, iodium, kali phosphoricum, phosphorus, ribes nigrum flower bud, soy lecithin, vitamin a acetate, vitamin e acetate solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63479-1404 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HERRING SPERM DNA (UNII: 51FI676N6F) (HERRING SPERM DNA - UNII:51FI676N6F) HERRING SPERM DNA 12 [hp_X] in 1 mL BOS TAURUS PLACENTA (UNII: 83AL37E3A7) (BOS TAURUS PLACENTA - UNII:83AL37E3A7) BOS TAURUS PLACENTA 7 [hp_C] in 1 mL IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 30 [hp_X] in 1 mL IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 30 [hp_X] in 1 mL DIBASIC POTASSIUM PHOSPHATE (UNII: CI71S98N1Z) (PHOSPHATE ION - UNII:NK08V8K8HR) DIBASIC POTASSIUM PHOSPHATE 30 [hp_X] in 1 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_X] in 1 mL RIBES NIGRUM FLOWER BUD (UNII: VYH9Y9BCCP) (RIBES NIGRUM FLOWER BUD - UNII:VYH9Y9BCCP) RIBES NIGRUM FLOWER BUD 8 [hp_X] in 1 mL LECITHIN, SOYBEAN (UNII: 1DI56QDM62) (LECITHIN, SOYBEAN - UNII:1DI56QDM62) LECITHIN, SOYBEAN 9 [hp_X] in 1 mL ALOE (UNII: V5VD430YW9) (ALOE - UNII:V5VD430YW9) ALOE 12 [hp_X] in 1 mL SUS SCROFA BONE MARROW (UNII: VP2CN2G7Y8) (SUS SCROFA BONE MARROW - UNII:VP2CN2G7Y8) SUS SCROFA BONE MARROW 7 [hp_C] in 1 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 6 [hp_X] in 1 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 6 [hp_X] in 1 mL BOS TAURUS SKIN (UNII: 7J12CD6O9L) (BOS TAURUS SKIN - UNII:7J12CD6O9L) BOS TAURUS SKIN 7 [hp_C] in 1 mL FUCUS VESICULOSUS (UNII: 535G2ABX9M) (FUCUS VESICULOSUS - UNII:535G2ABX9M) FUCUS VESICULOSUS 9 [hp_X] in 1 mL VITAMIN A ACETATE (UNII: 3LE3D9D6OY) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 9 [hp_X] in 1 mL .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) (ALPHA-TOCOPHEROL - UNII:H4N855PNZ1) .ALPHA.-TOCOPHEROL ACETATE 9 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63479-1404-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 07/15/1997 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/15/1997 Labeler - Apex Energetics Inc. (195816384)