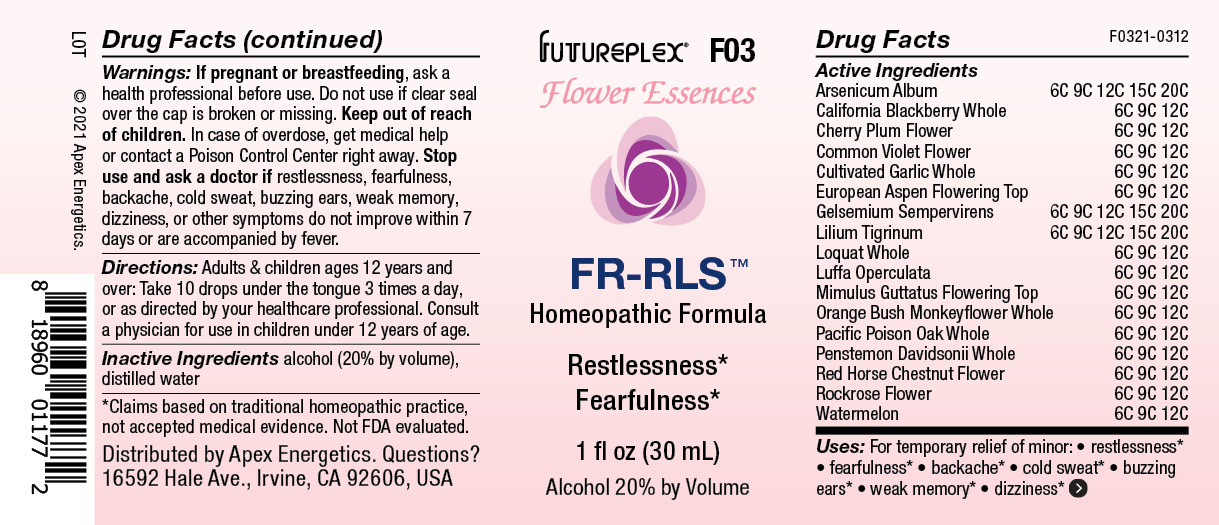
Label: F03 FR-RLS- aspen, arsenicum album, diplacus aurantiacus, garlic, lilium tigrinum, loquat, luffa operculata, mimulus guttatus, penstemon davidsonii, prunus cerasifera, red horse chestnut, rubus ursinus, rockrose, toxicodendron diversilobum, viola odorata, watermelon, yellow jasmine solution/ drops
- NDC Code(s): 63479-0603-1
- Packager: Apex Energetics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients
Arsenicum Album
6C 9C 12C 15C 20C
California Blackberry Whole
6C 9C 12C
Cherry Plum Flower
6C 9C 12C
Common Violet Flower
6C 9C 12C
Cultivated Garlic Whole
6C 9C 12C
European Aspen Flowering Top
6C 9C 12C
Gelsemium Sempervirens
6C 9C 12C 15C 20C
Lilium Tigrinum
6C 9C 12C 15C 20C
Loquat Whole
6C 9C 12C
Luffa Operculata
6C 9C 12C
Mimulus Guttatus Flowering Top
6C 9C 12C
Orange Bush Monkeyflower Whole
6C 9C 12C
Pacific Poisonoak Whole
6C 9C 12C
Penstemon Davidsonii Whole
6C 9C 12C
Red Horse-Chestnut Flower
6C 9C 12C
Rockrose Flower
6C 9C 12C
Watermelon
6C 9C 12C
- Uses:
- Warnings:
- Directions:
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
F03 FR-RLS
aspen, arsenicum album, diplacus aurantiacus, garlic, lilium tigrinum, loquat, luffa operculata, mimulus guttatus, penstemon davidsonii, prunus cerasifera, red horse chestnut, rubus ursinus, rockrose, toxicodendron diversilobum, viola odorata, watermelon, yellow jasmine solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63479-0603 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 20 [hp_C] in 1 mL VIOLA ODORATA FLOWER (UNII: 438W01FC7A) (VIOLA ODORATA FLOWER - UNII:438W01FC7A) VIOLA ODORATA FLOWER 12 [hp_C] in 1 mL GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 20 [hp_C] in 1 mL ERIOBOTRYA JAPONICA WHOLE (UNII: 2FCJ9QNJ7O) (ERIOBOTRYA JAPONICA WHOLE - UNII:2FCJ9QNJ7O) ERIOBOTRYA JAPONICA WHOLE 12 [hp_C] in 1 mL LUFFA OPERCULATA FRUIT (UNII: C4MO6809HU) (LUFFA OPERCULATA FRUIT - UNII:C4MO6809HU) LUFFA OPERCULATA FRUIT 12 [hp_C] in 1 mL MIMULUS GUTTATUS FLOWERING TOP (UNII: 192426I5JU) (MIMULUS GUTTATUS FLOWERING TOP - UNII:192426I5JU) MIMULUS GUTTATUS FLOWERING TOP 12 [hp_C] in 1 mL TOXICODENDRON DIVERSILOBUM WHOLE (UNII: 9211E5735B) (TOXICODENDRON DIVERSILOBUM WHOLE - UNII:9211E5735B) TOXICODENDRON DIVERSILOBUM WHOLE 12 [hp_C] in 1 mL PENSTEMON DAVIDSONII WHOLE (UNII: 8K9DCX606Z) (PENSTEMON DAVIDSONII WHOLE - UNII:8K9DCX606Z) PENSTEMON DAVIDSONII WHOLE 12 [hp_C] in 1 mL AESCULUS CARNEA FLOWER (UNII: 717DPT98VM) (AESCULUS CARNEA FLOWER - UNII:717DPT98VM) AESCULUS CARNEA FLOWER 12 [hp_C] in 1 mL RUBUS URSINUS WHOLE (UNII: EB3AN7DKSL) (RUBUS URSINUS WHOLE - UNII:EB3AN7DKSL) RUBUS URSINUS WHOLE 12 [hp_C] in 1 mL PRUNUS CERASIFERA FLOWER (UNII: 0KD7R09EAS) (PRUNUS CERASIFERA FLOWER - UNII:0KD7R09EAS) PRUNUS CERASIFERA FLOWER 12 [hp_C] in 1 mL ALLIUM SATIVUM WHOLE (UNII: IIF21WT8O3) (ALLIUM SATIVUM WHOLE - UNII:IIF21WT8O3) ALLIUM SATIVUM WHOLE 12 [hp_C] in 1 mL POPULUS TREMULA FLOWERING TOP (UNII: 5Q01F7TPJJ) (POPULUS TREMULA FLOWERING TOP - UNII:5Q01F7TPJJ) POPULUS TREMULA FLOWERING TOP 12 [hp_C] in 1 mL DIPLACUS AURANTIACUS WHOLE (UNII: TO6G4140YA) (DIPLACUS AURANTIACUS WHOLE - UNII:TO6G4140YA) DIPLACUS AURANTIACUS WHOLE 12 [hp_C] in 1 mL HELIANTHEMUM NUMMULARIUM FLOWER (UNII: 51BRR32WPP) (HELIANTHEMUM NUMMULARIUM FLOWER - UNII:51BRR32WPP) HELIANTHEMUM NUMMULARIUM FLOWER 12 [hp_C] in 1 mL WATERMELON (UNII: 231473QB6R) (WATERMELON - UNII:231473QB6R) WATERMELON 12 [hp_C] in 1 mL LILIUM LANCIFOLIUM WHOLE FLOWERING (UNII: X67Z2963PI) (LILIUM LANCIFOLIUM WHOLE FLOWERING - UNII:X67Z2963PI) LILIUM LANCIFOLIUM WHOLE FLOWERING 20 [hp_C] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63479-0603-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 03/12/1996 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/12/1996 Labeler - Apex Energetics Inc. (195816384)