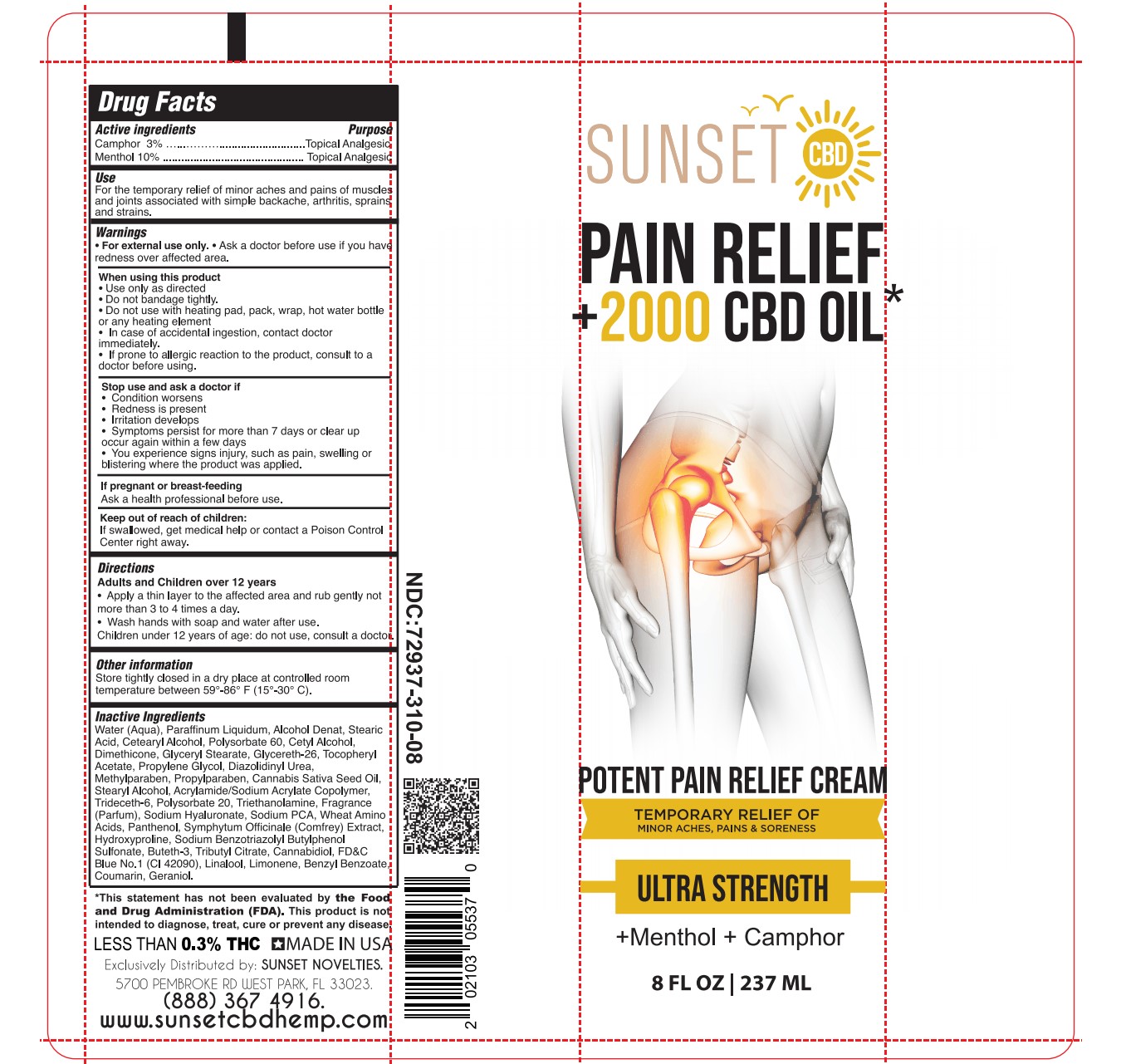
Label: MENTHOL, CAMPHOR cream
- NDC Code(s): 72937-310-08
- Packager: SUNSET NOVELTIES, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- Use
- WARNINGS
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
INACTIVE INGREDIENT
Water (Aqua), Paraffinum Liquidum, Alcohol Denat, Stearic Acid, Cetearyl Alcohol, Polysorbate 60, Cetyl Alcohol, Dimethicone, Glyceryl Stearate, Glycereth-26, Tocopheryl Acetate, Propylene Glycol, Diazolidinyl Urea, Methylparaben, Propylparaben, Cannabis Sativa Seed Oil, Stearyl Alcohol, Acrylamide/Sodium Acrylate Copolymer, Trideceth-6, Polysorbate 20, Triethanolamine, Fragrance (Parfum), Sodium Hyaluronate, Sodium PCA, Wheat Amino Acids, Panthenol, Symphytum Officinale (Comfrey) Extract, Hydroxyproline, Sodium Benzotriazolyl Butylphenol Sulfonate, Buteth-3, Tributyl Citrate, Cannabidiol, FD&C Blue No.1 (CI 42090), Linalool, Limonene, Benzyl Benzoate, Coumarin, Geraniol.
- SUNSET PAIN RELIEF CREAM TUBE 8 FL OZ
-
INGREDIENTS AND APPEARANCE
MENTHOL, CAMPHOR
menthol, camphor creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72937-310 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 10 g in 100 g CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 3 g in 100 g Inactive Ingredients Ingredient Name Strength LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) TRIBUTYL CITRATE (UNII: 827D5B1B6S) PANTHENOL (UNII: WV9CM0O67Z) HYDROXYPROLINE (UNII: RMB44WO89X) PROPYLPARABEN (UNII: Z8IX2SC1OH) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) ACRYLIC ACID/SODIUM ACRYLATE COPOLYMER (1:1; 600 MPA.S AT 0.2%) (UNII: M4PPW69Y4H) GLYCERETH-26 (UNII: NNE56F2N14) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) BUTETH-3 (UNII: OC116GRO69) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) WATER (UNII: 059QF0KO0R) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) METHYLPARABEN (UNII: A2I8C7HI9T) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) TRIDECETH-6 (UNII: 3T5PCR2H0C) POLYSORBATE 20 (UNII: 7T1F30V5YH) SODIUM BENZOTRIAZOLYL BUTYLPHENOL SULFONATE (UNII: 0LA2QC9O3Z) POLYSORBATE 60 (UNII: CAL22UVI4M) CETYL ALCOHOL (UNII: 936JST6JCN) DIMETHICONE 1000 (UNII: MCU2324216) ALCOHOL (UNII: 3K9958V90M) TROLAMINE (UNII: 9O3K93S3TK) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) COUMARIN (UNII: A4VZ22K1WT) CANNABIDIOL (UNII: 19GBJ60SN5) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) AMINO ACIDS, WHEAT (UNII: 0370GZL32F) BENZYL BENZOATE (UNII: N863NB338G) COMFREY (UNII: D05HXK6R3G) GERANIOL (UNII: L837108USY) MINERAL OIL (UNII: T5L8T28FGP) Product Characteristics Color green Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72937-310-08 226 g in 1 TUBE; Type 0: Not a Combination Product 03/04/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/04/2021 Labeler - SUNSET NOVELTIES, INC (067218145)