Label: IMPAVIDO- miltefosine capsule
- NDC Code(s): 69051-300-01
- Packager: Profounda, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 23, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use IMPAVIDO safely and effectively. See full prescribing information for IMPAVIDO.
IMPAVIDO (miltefosine) capsules, for oral use
Initial U.S. Approval: 2014WARNING: EMBRYO-FETAL TOXICITY
See full prescribing information for complete boxed warning.
- Pregnancy: IMPAVIDO is contraindicated in pregnancy. Based on animal data, miltefosine may cause fetal harm (4.1, 5.1, 8.1).
- Females of Reproductive Potential: Verify pregnancy status prior to initiating IMPAVIDO treatment in females of reproductive potential. To prevent pregnancy, females of reproductive potential should use effective contraception during IMPAVIDO treatment and for 5 months after the last dose (2, 5.1, 8.3, 13.1).
RECENT MAJOR CHANGES
Warnings and Precautions, Ocular Complications (5.10) 3/2025 INDICATIONS AND USAGE
IMPAVIDO is an antileishmanial drug indicated in adults and pediatric patients 12 years of age and older weighing greater than or equal to 30 kg (66 lbs) for the treatment of:
- •
- Visceral leishmaniasis due to Leishmania donovani (1).
- •
- Cutaneous leishmaniasis due to Leishmania braziliensis, Leishmania guyanensis, and Leishmania panamensis (1).
- •
- Mucosal leishmaniasis due to Leishmania braziliensis (1).
Limitations of use
Leishmania species evaluated in clinical trials were based on epidemiologic data. There may be geographic variation in the response of the same Leishmania species to IMPAVIDO (1, 14). The efficacy of IMPAVIDO in the treatment of other Leishmania species has not been evaluated.
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Each IMPAVIDO capsule for oral use contains 50 mg miltefosine (3).
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- Impaired Semen Quality and Spermatogenesis: Advise male patients that semen quality may be adversely affected after treatment with IMPAVIDO and that effects on spermatogenesis may persist for an unknown duration of time. IMPAVIDO may impair male fertility (5.2, 6.1, 8.3).
- •
- Female Reproductive Effects: IMPAVIDO caused impaired fertility in female rats. Advise females of reproductive potential of the reproductive toxicities found in animal studies. The potential effects of IMPAVIDO on female fertility have not been adequately evaluated (5.3, , 13.1)
- •
- Absorption of Oral Contraceptives: Advise females to use an additional non-oral method of effective contraception if vomiting and/or diarrhea occur (5.4,).
- •
- Renal Effects: Monitor serum creatinine during therapy and for 4 weeks after end of therapy (5.5, 6.1).
- •
- Hepatic Effects: Monitor transaminases and bilirubin during therapy (5.6, 6.1).
- •
- Gastrointestinal Effects: Encourage fluid intake (5.7).
- •
- Thrombocytopenia: Monitor platelet count during therapy for visceral leishmaniasis (5.8, 6.1).
- •
- Stevens-Johnson Syndrome: Discontinue IMPAVIDO (5.9).
- •
- Ocular Complications: Discontinue IMPAVIDO immediately if keratitis, keratopathy, acute scleritis, or anterior uveitis/iritis occur and consult an ophthalmologist (5.10).
ADVERSE REACTIONS
- •
- Adverse reactions occurring in ≥2% of patients include nausea, vomiting, diarrhea, headache, decreased appetite, dizziness, abdominal pain, pruritus, somnolence, elevated transaminases, and elevated creatinine (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Knight, at 1-844-483-5636 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: EMBRYO-FETAL TOXICITY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Pregnancy
4.2 Sjögren-Larsson-Syndrome
4.3 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
5.2 Impaired Semen Quality and Impaired Spermatogenesis
5.3 Female Reproductive Effects
5.4 Absorption of Oral Contraceptives
5.5 Renal Effects
5.6 Hepatic Effects
5.7 Gastrointestinal Effects
5.8 Thrombocytopenia
5.9 Stevens-Johnson Syndrome
5.10 Ocular Complications
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Treatment of Visceral Leishmaniasis
14.2 Treatment of Cutaneous Leishmaniasis
14.3 Treatment of Mucosal Leishmaniasis
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: EMBRYO-FETAL TOXICITY
- •
- Pregnancy: IMPAVIDO is contraindicated in pregnancy. Based on animal data, miltefosine may cause fetal harm [see Contraindications (4.1), Warnings and Precautions (5.1), and Use in Specific Populations (8.1)].
- •
- Females of Reproductive Potential: Verify pregnancy status prior to initiating IMPAVIDO. To prevent pregnancy, females of reproductive potential should use effective contraception during treatment and for 5 months after the last dose [see Dosage and Administration (2), Warnings and Precautions (5.1), Use in Specific Populations (8.3) and Nonclinical Toxicology (13.1)].
-
1 INDICATIONS AND USAGE
IMPAVIDO (miltefosine) capsules are indicated in adults and pediatric patients 12 years of age and older weighing greater than or equal to 30 kg (66 lbs) for the treatment of:
- •
- Visceral leishmaniasis caused by Leishmania donovani [see Clinical Trials (14.1)].
- •
- Cutaneous leishmaniasis caused by Leishmania braziliensis, Leishmania guyanensis, and Leishmania panamensis [see Clinical Trials (14.2)].
- •
- Mucosal leishmaniasis caused by Leishmania braziliensis [see Clinical Trials (14.3)].
Limitations of Use:
- •
- Leishmania species studied in clinical trials evaluating IMPAVIDO were based on epidemiologic data [see Clinical Trials (14.1, 14.2)].
- •
- There may be geographic variation in clinical response of the same Leishmania species to IMPAVIDO [see Clinical Trials (14.1, 14.2)].
- •
- The efficacy of IMPAVIDO in the treatment of other Leishmania species has not been evaluated.
-
2 DOSAGE AND ADMINISTRATION
Verify pregnancy status prior to initiating IMPAVIDO in females of reproductive potential [see Use in Specific Populations, (8.3)].
The treatment duration is 28 consecutive days. Administer with food to ameliorate gastrointestinal adverse reactions.
Table 1: IMPAVIDO Dosage Weight
Dosage and Administration
30 kg to 44 kg
One 50 mg capsule twice daily with food (breakfast and dinner)
45 kg or greater
One 50 mg capsule three times daily with food (breakfast, lunch, and dinner)
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Pregnancy
IMPAVIDO is contraindicated in patients who are pregnant. Based on animal data, miltefosine may cause fetal harm. [see Boxed Warning, Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
4.2 Sjögren-Larsson-Syndrome
IMPAVIDO is contraindicated in patients who have Sjögren-Larsson-Syndrome [see Clinical Pharmacology (12.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
IMPAVIDO is contraindicated in patients who are pregnant. Based on animal data, miltefosine may cause fetal harm. Embryo-fetal toxicity, including death and fetal malformations, was observed in animals administered miltefosine prior to mating, during early pregnancy, and during organogenesis at doses lower than the maximum recommended human dose (MRHD). Advise females of reproductive potential of the potential risk to a fetus. Verify pregnancy status prior to initiating IMPAVIDO in females of reproductive potential. Advise females of reproductive potential to use effective contraception during treatment with IMPAVIDO and for 5 months after the last dose [see Boxed Warning, Contraindications (4.1) and Use in Specific Populations (8.1, 8.3)].
5.2 Impaired Semen Quality and Impaired Spermatogenesis
IMPAVIDO may impair male fertility. Reductions in semen parameters (ejaculate volume, total sperm count, sperm concentration, sperm morphology, sperm motility) were observed in a clinical study evaluating the effects of IMPAVIDO on spermatogenesis. For all parameters, except sperm concentration, the observed reductions were reversible in most affected patients and improved within 3 to 6 months. Reductions in sperm concentration of > 50% persisted in up to 26% of patients. Reductions up to the lower limit of normal in sperm concentration (< 20 million/ mL) persisted in up to 8% of patients. Per protocol, semen parameters were not assessed beyond 6 months in any patient. The effect of IMPAVIDO on spermatogenesis may persist for an unknown duration. [see Adverse Reactions (6.1) and Use in Specific Populations (8.3)].
Reductions in ejaculate volume, temporary absence of ejaculate, and scrotal tenderness were reported in an observational study of male patients who received IMPAVIDO. These adverse reactions resolved in all patients upon completion of IMPAVIDO therapy [see Adverse Reactions (6.2)].
5.3 Female Reproductive Effects
IMPAVIDO caused impaired fertility in female rats and follicular atresia and reversible anestrus/diestrus in dogs at doses approximately 1.0 and 0.2 times the MRHD based on body surface area comparisons, respectively [see Nonclinical Toxicology (13.1)]. The effects of IMPAVIDO on human female fertility have not been formally studied [see Use in Specific Population (8.3)].
5.4 Absorption of Oral Contraceptives
Vomiting and/or diarrhea occurring during IMPAVIDO therapy may affect the absorption of oral contraceptives, and therefore compromise their efficacy. If vomiting and/or diarrhea occur during IMPAVIDO therapy, advise females to use an additional non-oral method of effective contraception [see Boxed Warning, Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
5.5 Renal Effects
Elevations of serum creatinine (Cr) were noted in clinical trials evaluating IMPAVIDO in the treatment of cutaneous, mucosal and visceral leishmaniasis. Monitor renal function weekly in patients receiving IMPAVIDO during therapy and for 4 weeks after end of therapy [see Adverse Reactions (6.1)].
5.6 Hepatic Effects
Elevations in liver transaminases (ALT, AST) and bilirubin were noted in clinical trials evaluating IMPAVIDO in the treatment of visceral leishmaniasis. Monitor liver transaminases (ALT, AST) and bilirubin during therapy in patients receiving IMPAVIDO [see Adverse Reactions (6.1)].
5.7 Gastrointestinal Effects
Vomiting and/or diarrhea commonly occur during IMPAVIDO administration and may result in volume depletion. Encourage fluid intake to avoid volume depletion [see Adverse Reactions (6.1)].
5.8 Thrombocytopenia
Thrombocytopenia during therapy has been reported in patients treated for visceral leishmaniasis. Monitor platelet count during therapy for visceral leishmaniasis [see Adverse Reactions (6.1, 6.2)].
5.9 Stevens-Johnson Syndrome
Stevens-Johnson syndrome has been reported during IMPAVIDO therapy. Discontinue IMPAVIDO if an exfoliative or bullous rash is noted during therapy [see Adverse Reactions (6.1)].
5.10 Ocular Complications
Ocular complications including keratitis, keratopathy, acute scleritis, and anterior uveitis/iritis have been reported in patients treated with IMPAVIDO.
Ocular complications of IMPAVIDO therapy have been reported during the treatment of all forms of leishmaniasis (cutaneous, mucosal, and visceral) and during use of IMPAVIDO for conditions other than cutaneous, mucosal and visceral leishmaniasis. Ocular complications are also known to occur in patients with leishmaniasis.
Keratitis, keratopathy, acute scleritis, and anterior uveitis/iritis have been reported both within a few days of and after several weeks of IMPAVIDO therapy. While these ocular complications have most commonly been reported in individuals being treated for post-kala-azar dermal leishmaniasis, it is not known whether the higher frequency of reports is due to the particular form of leishmaniasis or the typically longer duration of treatment which occurs with the treatment of postkalaazar dermal leishmaniasis. The safety and effectiveness of IMPAVIDO in the treatment of post-kala-azar dermal leishmaniasis has not been established.
Consideration should be given to having patients receive a baseline eye exam before starting IMPAVIDO.
The most commonly reported ocular signs and symptoms after starting IMPAVIDO include: red eye, eye pain, photophobia, increased lacrimation, decreased visual acuity, corneal ulceration, corneal opacities, and partial or complete blindness which in some cases has been permanent.
Instruct patients to discontinue IMPAVIDO immediately and follow-up with an ophthalmologist if they develop an ocular adverse reaction while on IMPAVIDO. Treatment with ocular anti-inflammatory agents may be warranted.
If it is determined that significant eye findings occurred as a result of treatment with IMPAVIDO therapy, initiate an alternative anti-leishmanial treatment. IMPAVIDO has a long half-life [see Clinical Pharmacology (12.3)], therefore, it is possible, even with discontinuation of IMPAVIDO therapy, that ocular changes may not resolve.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Clinical Trials Experience
Visceral Leishmaniasis
One Phase 3 trial was conducted in patients ≥ 12 years of age in India. Two-hundred and ninety-nine (299) patients (211 men and 88 women) received oral IMPAVIDO at a target dose of 2.5 mg/kg/day for 28 days (50 mg capsule once daily if weight was less than 25 kg and 50 mg capsule twice daily if weight was 25 kg or greater). Patients ranged between 12 and 64 years of age. Weight ranged between 15 and 67 kg (mean weight 38.6 kg) and BMI ranged between 8.2 and 24 (mean 16.1). Ninety-nine (99) patients received 1 mg/kg/day amphotericin B deoxycholate intravenously every other day for 15 doses. A statistically significant higher percentage of men received IMPAVIDO compared to amphotericin B.
Less than 1% of patients who received IMPAVIDO died (2/299) and no patient who received amphotericin B died. Serious adverse reactions were reported in 2% of IMPAVIDO recipients (6/299) and 1% of amphotericin B recipients (1/99). Approximately 3% of patients discontinued treatment in each treatment arm due to an adverse reaction. Serious adverse reactions and adverse reactions leading to drug discontinuation that were thought to be related or possibly related to IMPAVIDO included Stevens-Johnson syndrome, melena and thrombocytopenia, arthritis and skin rash, Common Terminology Criteria for Adverse Events (CTCAE) Grade 4 diarrhea (≥10 stools per day) and CTCAE Grade 4 hyperbilirubinemia (≥10x upper limit of normal ULN).
Table 2: Treatment Emergent Adverse Reactions Occurring in ≥2% of Visceral Leishmaniasis Patients Receiving IMPAVIDO System Organ Class
Preferred TermIMPAVIDO
N = 299Amphotericin B Deoxycholate
N = 99Gastrointestinal Disorders
Diarrhea
61 (20.4%)
6 (6.1%)
Vomiting
113 (37.8%)
20 (20.0%)
General Disorders
Asthenia
19 (6.3%)
4 (4.0%)
Metabolism and Nutrition Disorders
Decreased Appetite
69 (23.1%)
22 (22.2%)
In this study, creatinine (Cr) elevations ≥ 1.5 times above baseline occurred in approximately 10% of IMPAVIDO recipients and in 40% of amphotericin B recipients at the end of therapy. Ten percent of subjects in each arm had Cr elevations ≥1.5 times above baseline at 6 months follow up. No IMPAVIDO recipient discontinued therapy due to Cr elevation.
Elevations of transaminases during therapy occurred in up to half of IMPAVIDO recipients and up to a third of amphotericin B recipients. The elevations were mild (< 3x ULN) or moderate (3-5x ULN) in 94% and 6% respectively of IMPAVIDO-treated patients who experienced an elevation. No patient discontinued therapy due to elevations in transaminases.
At the end of therapy, 62% and 2.4% of IMPAVIDO recipients and 54% and 2% of amphotericin B recipients had platelet count < 150,000 and < 50,000 respectively.
Cutaneous Leishmaniasis
The efficacy of IMPAVIDO in the treatment of cutaneous leishmaniasis was evaluated in one placebo-controlled trial conducted in Colombia and Guatemala and in two comparative trials conducted in Bolivia and Brazil respectively. In the placebo-controlled trial, eighty-nine (89) patients 12 years of age and older received a target IMPAVIDO dose of 2.5 mg/kg/day for 28 days and forty-four (44) received placebo. In the comparative trials, one hundred and twenty (120) patients 12 years of age and older received a target IMPAVIDO dose of 2.5 mg/kg/day for 28 days and fifty-eight (58) patients received 20 mg/kg/day pentavalent antimony (meglumine) parenterally for 20 days.
Table 3: Adverse Reactions Occurring in ≥2% of IMPAVIDO-Treated Patients ≥12 Years of Age with Cutaneous Leishmaniasis in the Placebo-Controlled Trial System Organ Class
Adverse ReactionIMPAVIDO
N = 89Placebo
N = 44Ear and Labyrinth Disorders
Motion Sickness
26 (29.2%)
10 (22.7%)
Gastrointestinal Disorders
Abdominal Pain
10 (11.2%)
3 (6.8%)
Diarrhea
7 (7.9%)
2 (4.5%)
Nausea
32 (35.9%)
5 (11.1%)
Vomiting
4 (4.5%)
0
General and Administration Site Disorders
Malaise
3 (3.4%)
1 (2.3%)
Pyrexia
5 (5.6%)
2 (4.5%)
Nervous System Disorders
Dizziness
4 (4.5%)
0
Headache
25 (28.1%)
10 (22.7%)
Somnolence
3 (3.4%)
0
Skin and Subcutaneous Tissue Disorders
Pruritus
4 (4.5%)
0
Table 4: Adverse Reactions Occurring in ≥2% of IMPAVIDO-Treated Patients ≥ 12 Years of Age with Cutaneous Leishmaniasis in Two Comparative Trials System Organ Class
Adverse ReactionIMPAVIDO
N = 120Meglumine
N = 58Gastrointestinal Disorders
Abdominal Pain
9 (7.5%)
3 (5.2%)
Diarrhea
18 (15.0%)
3 (5.2%)
Nausea
50 (41.7%)
3 (5.2%)
Vomiting
33 (27.5%)
0
Infections and Infestations
Lymphangitis
7 (5.8%)
0
Metabolism and Nutrition Disorders
Decreased Appetite
13 (10.8%)
4 (5.8%)
Nervous System Disorders
Dizziness
15 (12.5%)
4 (6.9%)
Skin and Subcutaneous Tissue Disorders
Pruritus
7 (5.8%)
0
In the placebo-controlled trial, 12/89 (13.4%) IMPAVIDO subjects had Cr increases of 1.5-3 times above baseline, compared to 2/44 (4.5%) placebo subjects at end of therapy. In the comparative trial, a similar percentage of subjects who received IMPAVIDO or pentavalent antimony had Cr elevations above baseline at 3 and 6 months after therapy (approximately 5%). Approximately 25% of IMPAVIDO subjects and 11% of pentavalent antimony subjects had Cr elevations 1.5-3 times above baseline at the end of therapy in the two active controlled trials. The frequency of AST and ALT increase above upper limit of normal at end of therapy was similar in IMPAVIDO and placebo recipients (approximately 5%).
Other adverse events seen at <2% incidence in the IMPAVIDO group included anemia, lymphadenopathy, abdominal distension, constipation, dysphagia, flatulence, fatigue, malaise, abscess, cellulitis, ecthyma, paresthesia, testicular pain, testicular swelling, Stevens-Johnson syndrome, urticaria, rash, pyoderma.
Adverse Effects on Semen Quality and Spermatogenesis
In an open-label, uncontrolled, single-center study that assessed the effects of IMPAVIDO on sperm parameters, a total of 58 Bolivian adult males with cutaneous or mucosal leishmaniasis were administered IMPAVIDO for 28 days at a target dose of 2.5 mg/kg/day. Patients underwent repeat semen analysis testing at baseline (prior to treatment), at the end of treatment (Day 25 to 28 of treatment), and at 3 months after completing treatment. If sperm concentrations were markedly reduced at 3 months, per protocol, one additional semen sample was collected at 6 months after completing treatment.
The primary safety endpoint was determined at the end of treatment and included the number and percentage of patients with abnormal sperm parameters and clinically relevant changes from baseline in semen volume, total sperm count, sperm concentration, sperm motility and sperm morphology as shown in Table 5. The secondary safety endpoints included: mean changes from baseline in semen volume, sperm concentration, total sperm count, sperm motility and sperm morphology. Mean changes from baseline in serum testosterone and FSH concentrations were also evaluated.
A total of 53 patients completed the study. The median patient age was 34 years (range 18 to 51 years). The majority of patients (70.7%) had cutaneous leishmaniasis, while 24.1% had mucosal leishmaniasis, and 5.2% had both forms.
Treatment with IMPAVIDO was associated with reductions in all sperm parameters at the end of treatment (see Table 5). All sperm parameter reductions, except for sperm concentration, recovered on follow-up assessments at 3 and 6 months after treatment completion. For sperm concentration, small mean decreases persisted on follow-up assessments at 3 and 6 months after treatment completion, with approximately 26% of subjects showing post-treatment sperm concentrations reductions of ≥ 50%, and 8% showing reductions to the lower limit of normal (< 20 million/ mL), on their last observed assessment.
Table 5 shows the results for the primary safety endpoint, the number and percentage of patients with abnormal sperm parameters at the end of treatment and in the post-treatment follow-up period, including assessments at 3 months or 6 months after treatment completion.
Table 5: Number (%) of Subjects with Abnormal Sperm Parameters, Based on Last Observation, n=55 - *
- Last observation=Patient’s final semen analysis at either 3 months or 6 months after treatment completion
Sperm Parameter
End of Treatment
Follow-up Period
(Last observation*)Semen volume
< 1.5 mL
41/55 (75%)
8/53 (15%)
Total sperm count
≥ 50% reduction from baseline
28/52 (54%)
10/53 (19%)
< 39 million
17/52 (33%)
3/53 (6%)
Sperm concentration
≥ 50% reduction from baseline
11/52 (21%)
14/53 (26%)
< 20 million sperm/mL
6/52 (12%)
4/53 (8%)
Sperm motility
≥ 25% reduction from baseline
28/55 (51%)
5/53 (9%)
< 40% with total motility
18/55 (33%)
3/53 (6%)
Sperm morphology
≥ 25% reduction from baseline
17/55 (31%)
8/53 (15%)
< 4% normal forms
0/55 (0%)
0/53 (0%)
Semen analyses were not conducted beyond 6 months in any patient; therefore, the duration of effect of IMPAVIDO on sperm concentration after treatment is unknown.
Table 6 shows the results for the secondary safety endpoints, mean sperm parameters at baseline, end of treatment, and 3 and 6 months after treatment completion.
Table 6: Mean Sperm Parameters Over Time * If sperm concentrations were markedly reduced at 3 months, per protocol, one additional semen sample was collected at 6 months after completing treatment.
** Mean and SD of log(X+1) transformed data are calculated and transformed back to original unit using exp(X)-1 to derive Geometric Mean and Geometric SD in this table.n
Geometric Mean (SD)**
Semen Baseline
55
2.3 (0.4)
Volume
End of Treatment
55
1.0 (0.4)
(mL)
3 Months
49
2.1 (0.3)
6* Months
18
2.3 (0.4)
Total Sperm
Baseline
55
169 (1.0)
Count
End of Treatment
52
74 (3.8)
(millions)
3 Months
49
128 (1.3)
6* Months
18
129 (1.9)
Sperm
Baseline
55
79 (1.2)
Concentration
End of Treatment
52
74 (2.8)
(million/mL)
3 Months
49
65 (1.3)
6* Months
18
59 (1.5)
n
Mean (SD)
Sperm Motility
Baseline
55
59 (8.8)
(%)
End of Treatment
55
39 (15.2)
3 Months
49
57 (10.4)
6* Months
18
54 (10.6)
Sperm Morphology
Baseline
55
7 (4.2)
(%)
End of Treatment
52
15 (5.1)
3 Months
49
18 (5.7)
6* Months
18
16 (5.5)
No clinically meaningful changes were observed in serum testosterone or FSH concentrations at the end of treatment or 3 months after treatment.
6.2 Postmarketing Experience
The following adverse reactions have been identified during use of IMPAVIDO or miltefosine worldwide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse Reactions from Postmarketing Spontaneous Reports
Blood and Lymphatics Disorders: thrombocytopenia, agranulocytosis
Eye Disorders: keratitis, keratopathy, and acute scleritis; uveitis
Gastrointestinal Disorders: melena
General Disorders: generalized edema, peripheral edema
Hepatobiliary Disorders: jaundice
Nervous System Disorders: seizure
Vascular Disorders: epistaxis
Adverse Reactions from Observational Studies
Reproductive System and Breast Disorders: scrotal pain, decreased ejaculate volume, absent ejaculation.
Investigations: uric acid elevations
Musculoskeletal disorders: acute gout
Reduced Ejaculate Volume and Scrotal Tenderness
Among 33 young male patients treated with miltefosine at a single Dutch center, 21 (64%) reported diminution of ejaculate volume and 2 (6%) reported temporary absence of ejaculate. In addition, 4 patients (12%) reported scrotal tenderness and 1 (3%) was diagnosed with epididymitis. These adverse reactions resolved in all patients upon completion of their miltefosine therapy.
-
7 DRUG INTERACTIONS
In vitro and animal metabolism studies showed that miltefosine did not markedly induce or inhibit the activity of the major human cytochrome P450 enzymes [see Clinical Pharmacology (12.3)]. The potential of miltefosine to interact with drug transporters has not been evaluated.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to IMPAVIDO during pregnancy. Healthcare providers are encouraged to register patients by calling 1-866-588-5405.
Risk Summary
IMPAVIDO is contraindicated during pregnancy. Based on data from animal reproduction studies, IMPAVIDO may cause embryo-fetal toxicity when administered to pregnant women. There are no available data on IMPAVIDO use in pregnant women to determine a drug-associated risk of major birth defects, miscarriage, or adverse maternal and/or fetal outcomes. If a woman becomes pregnant while being treated with IMPAVIDO, treatment should be discontinued and the patient should be counseled about the potential risk to the fetus.
Embryo-fetal toxicity, including death and fetal malformations, was observed in embryo-fetal studies in rats and rabbits administered oral miltefosine during organogenesis at doses that were respectively 0.06 and 0.2 times the maximum recommended human dose (MRHD) of 3.33 mg/kg/day. Numerous visceral and skeletal fetal malformations were observed in a fertility study in female rats administered miltefosine prior to mating through day 7 of pregnancy at doses 0.3 times the MRHD.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In an embryo-fetal development study in pregnant rats, miltefosine was administered in oral doses of 0.6, 1.2, 2.4, 6, 12, and 24 mg/kg/day during the period of organogenesis (Day 6 to Day 15 of gestation). Miltefosine dosages ≥ 1.2 mg/kg/day [0.06 times the MRHD based on body surface area (BSA) comparison] caused embryo-fetal toxicity including death and fetal malformations. Malformations included undeveloped cerebrum, hemorrhagic fluid filling the lumina of the skull, cleft palate and generalized edema. In an embryo-fetal development study in pregnant rabbits, miltefosine was administered in oral doses of 0.6, 1.2, 2.4, 6, 12, and 24 mg/kg/day during the period of organogenesis (Day 6 to Day 18 of gestation). Abortion and fetal resorption occurred with one dam receiving 2.4 mg/kg/day miltefosine (0.2 times the MRHD based on BSA comparison) and fetal resorptions occurred with all dams receiving ≥ 6.0 mg/kg/day miltefosine. In both rats and rabbits, there were no viable litters at miltefosine doses ≥ 6.0 mg/kg/day (0.3 or 0.6 times the MRHD based on BSA comparisons for rats and rabbits respectively).
In a separate fertility study in female rats, miltefosine was administered in oral doses of 2.15, 6.81, and 21.5 mg/kg for four weeks before mating and up to Day 7 of pregnancy. Miltefosine doses ≥ 6.81 mg/kg/day (0.3 times the MRHD based on BSA comparison) produced numerous visceral (misshapen cerebral structures, dilated ventricles filled with brown masses, misshapen spinal cord, misshapen and malpositioned eyes, hypophysis, and absent inner ear) and skeletal (cleft palate, dumbbell-shaped ossification of thoracic vertebral centers, markedly enlarged skull bones, and markedly dilated suturae) fetal malformations. [see Contraindications (4.1), Nonclinical Toxicology (13.1)].
8.2 Lactation
Risk Summary
There are no data on the presence of miltefosine in human or animal milk, the effects on the breastfed infants, or the effects on milk production. Because of the potential for serious adverse reactions, breastfeeding is not recommended during treatment with IMPAVIDO and for 5 months after the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status prior to initiating IMPAVIDO in females of reproductive potential [see Boxed Warning and Use in Specific Populations (8.1)].
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with IMPAVIDO and for 5 months after the last dose [see Boxed Warning, Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
Vomiting and/or diarrhea occurring during IMPAVIDO therapy may affect absorption of oral contraceptives and therefore compromise their efficacy. If vomiting and/or diarrhea occur during IMPAVIDO therapy, advise females to use an additional non-oral method of effective contraception [see Boxed Warning, Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
Infertility
Females
Based on animal fertility studies, IMPAVIDO may impair fertility in females of reproductive potential [see Nonclinical Toxicology (13.1)]. The effects of IMPAVIDO on human female fertility have not been formally studied [see Warnings and Precautions (5.3)].
Males
Based on animal fertility and postmarketing studies, IMPAVIDO may impair fertility in males of reproductive potential [see Warnings and Precautions (5.2) and Nonclinical Toxicology (13.1)].
In an open-label, uncontrolled, single-center study that assessed the effects of miltefosine on sperm parameters, a total of 58 adult males with cutaneous or mucosal leishmaniasis were administered miltefosine for 28 days at a target dose of 2.5 mg/kg/day and underwent semen analysis testing prior to treatment, at the end of treatment, at 3 months after completing treatment, and if needed, at 6 months after completing treatment. The primary safety endpoint was determined at the end of treatment and included the number and percentage of patients with abnormal sperm parameters and clinically relevant changes from baseline in sperm parameters. The secondary safety endpoints included mean sperm parameters at baseline, end of treatment, and 3 and 6 months after treatment completion. Mean serum testosterone and FSH concentrations were also evaluated [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
Treatment with IMPAVIDO was associated with reductions in all sperm parameters at the end of treatment. All sperm parameters, except for sperm concentration, recovered on follow-up assessments at 3 and 6 months after treatment completion [see Adverse Reactions (6.1)].
For sperm concentration, small mean decreases persisted on follow-up assessments at 3 and 6 months after treatment completion, with approximately 26% of subjects showing post-treatment sperm concentrations reductions of ≥ 50% and reductions to the lower limit of normal (< 20 million/ mL) persisting in up to 8% of patients on their last observed assessment. Semen analyses were not conducted beyond 6 months in any patient, therefore, the duration of effect of IMPAVIDO on sperm concentration after treatment is unknown [see Adverse Reactions (6.1)].
No clinically meaningful changes were observed in serum testosterone or FSH concentrations.
Reductions in ejaculate volume and temporary absence of ejaculate were reported in an observational study of male patients who received IMPAVIDO. These adverse reactions resolved in all patients in this study upon completion of IMPAVIDO therapy [see Warnings and Precautions (5.2) and Adverse Reactions (6.2)].
The effect of IMPAVIDO on spermatogenesis may persist for an unknown duration.
Whether IMPAVIDO affects male fertility is unknown [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
8.4 Pediatric Use
Safety and effectiveness in pediatric patients less than 12 years old have not been established. Juvenile rats were more sensitive to the miltefosine-induced effects, especially retinal and kidney effects, than adult rats [see Indications and Usage (1)].
8.5 Geriatric Use
Clinical studies of IMPAVIDO did not include sufficient numbers of subjects 65 years of age and over to determine if they respond differently than younger subjects.
-
10 OVERDOSAGE
The common adverse effects of vomiting, diarrhea, and abdominal pain are likely in case of overdose. Institute adequate hydration to prevent the risk of impaired renal function and replace electrolytes as necessary. Because miltefosine is only slightly excreted in the urine, forced diuresis will not increase miltefosine excretion. Gastrointestinal lavage is of unknown value. A specific antidote to treat miltefosine overdose is not known.
-
11 DESCRIPTION
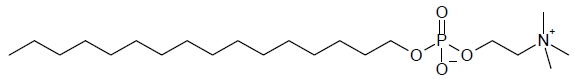
IMPAVIDO capsules contain the active ingredient miltefosine, an antileishmanial agent. The chemical name of miltefosine is 2-[[(hexadecyloxy)hydroxyphosphenyl]oxy]-N,N,N-trimethylethylammonium inner salt. Miltefosine is a white powder that is freely soluble in water, 0.1 N HCl or NaOH, methanol, and ethanol. It has the empirical formula of C21H46NO4P with a molecular weight of 407.6 and the following structural formula:
The inactive ingredients are colloidal silicon dioxide, microcrystalline cellulose, lactose monohydrate, talc, and magnesium stearate. The capsule shell contains gelatin, titanium dioxide, ferric oxide, and purified water.
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Cardiac Electrophysiology:
The effect of IMPAVIDO on the QTc interval was evaluated in 42 adult patients administered the recommended dosage regimen of IMPAVIDO capsules, 50 mg three times daily for 28 days.
No large increases (i.e., 20 msec) or any other evidence of prolongation of the corrected QTc interval (QTcF) from baseline were observed.
12.3 Pharmacokinetics
The pharmacokinetic parameters of miltefosine in patients with visceral and cutaneous leishmaniasis treated for 28 days with IMPAVIDO are listed in Table 7. Due to the long half-life of miltefosine (> 6 days), trough plasma concentrations did not appear to reach a steady state at the end of treatment (i.e., Day 28).
Table 7: Mean (%CV) Pharmacokinetic Parameters for Miltefosine Following Oral Capsule Administration to Adult Patients with Visceral and Cutaneous Leishmaniasis a: Adolescent (≥12 years)/Adults, mean dose per kg was 3.1 mg/kg/day
b: Adolescent (≥12 years)/Adults, mean dose per kg was 3.6 mg/kg/day
c: Adults, mean dose per kg was 1.8 mg/kg/day
d: median (range)
e: AUC0-12h for BID, AUC0-8h for TID
f: t1/2,α = distribution phase half-life; t1/2,β = terminal elimination phase half-life
g: Estimates based on a population PK model
h: mean (% standard error)Dose
Cmax
(µg/mL)Tmaxd
(hr)AUCtaue
(µg∙hr/mL)t1/2,αf
(day)t1/2,βg
(day)Visceral
Leishmaniasis
(on Day 23)50 mg BID (4 wks)a
66.2 (28.5)
7(2-12)
636 (26.7)
6.4
(31.1)50 mg BID (1 wk)/
50 mg TID (3 wks)b75.9 (17.6)
4 (2-8)
486 (18.1)
8.5
(28.9)Cutaneous
Leishmaniasisc
(on Day 27)50 mg TID (4 wks)
37.3 (22)f
295 (22)f
6.8
(5.8)g,h30.7
(18.3)g,hAbsorption
Absolute bioavailability of miltefosine has not been determined. In patients with visceral leishmaniasis, maximum miltefosine concentrations following oral administration of IMPAVIDO capsules were reached right before the next dose in many patients, indicating that the absorption of miltefosine may proceed throughout the dosing interval.
Distribution
The distribution of miltefosine has not been studied in humans. Human plasma protein binding of miltefosine, evaluated by an ultracentrifugation method, was 98% over the drug concentration range from 0.1 to 10 µg/mL. In rats, radioactivity of [14C] miltefosine is widely distributed after both single and repeated oral administration with highest uptake of radioactivity in kidney, liver, and spleen. Placental transfer and excretion into milk have not been investigated.
Metabolism and Excretion
No in vitro oxidative metabolism by 15 different human cytochrome P450 enzymes (1A1, 1A2, 1B1, 2A6, 2B6, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 3A4, 3A5, 3A7, and 4A1) was observed.
A slow metabolic breakdown could be shown in human hepatocytes, resulting in the release of choline by phospholipase D-like cleavage of the miltefosine molecule. The fatty alcohol-containing fragment of miltefosine can enter the metabolism of fatty acids after being oxidized to palmitic acid. This oxidation is blocked in patients with Sjögren-Larsson syndrome, which is caused by a genetic defect in fatty aldehyde dehydrogenase activity. IMPAVIDO is contraindicated in patients who have Sjögren-Larsson-Syndrome [see Contraindications (4.2)].
There was little or no evidence of time or metabolism dependent inhibition of the cytochrome P450 enzymes examined at up to approximately 40 µg/mL miltefosine.
Oral administration of miltefosine did not markedly induce the content of hepatic CYP3A assayed by demethylation activity of erythromycin in rats.
In visceral leishmaniasis patients, <0.2% of the administered dose was excreted into the urine.
12.4 Microbiology
Mechanism of Action
The specific mode of action of miltefosine against Leishmania species is unknown. The mechanism of action of miltefosine is likely to involve interaction with lipids (phospholipids and sterols), including membrane lipids, inhibition of cytochrome c oxidase (mitochondrial function), and apoptosis-like cell death.
Activity In Vitro and In Vivo
Miltefosine has anti-leishmanial activity in vitro and in clinical infections [see Clinical Studies (14)]. Sensitivity of different Leishmania species as well as different strains of a Leishmania species to miltefosine may vary in different geographic regions.
Drug Resistance
In vitro studies show a potential for development of resistance to miltefosine. Some strains of L. braziliensis with intrinsic resistance to miltefosine have been identified. However, the clinical relevance of these observations is not known.
Drug resistance could be due to a decrease in miltefosine accumulation within Leishmania parasite which is thought to be due to either an increase in drug efflux, mediated by the overexpression of the ABC transporter P-glycoprotein and/or a decrease in drug uptake by the inactivation of the miltefosine transport machinery that consists of the miltefosine transporter and its beta subunit. Mutation in the transporter gene was reported in the isolates from a relapsed patient in one study. However, the clinical relevance of these findings is not known.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenicity/Carcinogenicity: Miltefosine tested negative in the AMES-Salmonella test, DNA-amplification test, chromosomal aberration test in vitro, UDS-test in vivo/in vitro, and oral mouse micronucleus test in vivo. The V 79 mammalian cell HPRT gene mutation test showed an increase in mutant frequency without dose dependency. In view of all mutagenicity test results, the single positive finding in the V 79 HPRT test is considered to be not of toxicological relevance with respect to a mutagenic risk to humans.
Carcinogenicity studies were not performed. In a 52-week oral rat toxicity study, benign and malignant neoplasms that occurred in high-dose animals administered 21.5 mg/kg/day miltefosine (1.0 times the MRHD based on BSA comparison) included testicular Leydig cell adenoma in 3 of 30 male rats, and histiocytic sarcoma with widespread multi-organ metastasis, squamous cell carcinoma in the uterus, and a malignant adenoacanthoma in the uterus with widespread metastasis in 3 individual female rats. The carcinogenic potential of miltefosine in humans is unknown.
In a fertility study in male rats, miltefosine was administered in oral doses of 3.16, 8.25 and 21.5 mg/kg/day from 4 weeks prior to mating until 7 days during mating. Testicular atrophy, reduced numbers of viable sperm, and impaired fertility were observed in rats following daily oral doses of ≥ 8.25 mg/kg (0.4 times the MRHD based on BSA comparison). These findings were reversible within a recovery period of 10 weeks except at the highest dose tested, 21.5 mg/kg/day (1.0 times the MRHD based on BSA comparison), where effects were not fully reversible.
In a fertility study in female rats, miltefosine was administered in oral doses of 2.15, 6.81, and 21.5 mg/kg/day for 4 weeks prior to mating, during mating, and until Day 7 of pregnancy. Estrus cycle arrest in the metestrus or diestrus phases occurred with the high dose of 21.5 mg/kg (1.0 times the MRHD based on BSA comparison). At doses of 6.81 and 21.5 mg/kg (0.3 and 1.0 times the MRHD, respectively, based on BSA comparison) increased numbers of embryonic and fetal resorptions and dead fetuses were observed.
In a 52-week toxicology study in dogs, increased numbers of atretic follicles in the ovaries, and cycle arrest in the uterus, vagina, and mammary gland with morphology consistent with anestrus or diestrus were observed at doses ≥ 1 mg/kg/day (0.2 times the MRHD based on BSA comparison). The effects in dogs were fully reversible after a recovery period of 6 weeks.
-
14 CLINICAL STUDIES
14.1 Treatment of Visceral Leishmaniasis
One randomized, open-label, active-controlled study was conducted to evaluate the efficacy of IMPAVIDO in the treatment of visceral leishmaniasis in Bihar, India, an area where L. donovani is known epidemiologically to be the prevalent infecting species. Patients 12 years of age and older with clinical signs and symptoms compatible with visceral leishmaniasis (fever, splenomegaly, and cytopenia) confirmed by the presence of Leishmania amastigotes in aspirates of spleen or bone marrow were randomized to receive oral IMPAVIDO or intravenous amphotericin B deoxycholate in a 3:1 ratio. Patients weighing greater than or equal to 25 kg received an IMPAVIDO 50 mg capsule with meals twice a day. Patients weighing less than 25 kg received an IMPAVIDO 50 mg capsule with meals once a day in the morning. Weight ranged between 15 and 67 kg (mean weight 38.6 kg) and BMI ranged between 8.2 and 24 (mean 16.1). No patient weighed more than 70kg. Amphotericin B was administered intravenously over 6 continuous hours at 1 mg/kg every other day for 15 doses. Patients were hospitalized for the duration of therapy.
Exclusion criteria included platelet count <50 × 109/L, white cell count <1 × 109/L, hemoglobin <6 g/100 mL, AST or ALT ≥3 times upper limit of the normal range, bilirubin ≥2 times upper limit of the normal range, serum creatinine or BUN >1.5 times upper limit of the normal range, prothrombin time >5 seconds above control, and any non-compensated or uncontrolled condition including human immunodeficiency virus (HIV) infection. Women of reproductive potential were required to use effective contraception for the duration of therapy and for 2 months post therapy.
Final cure was defined as initial cure at end of therapy plus absence of signs and symptoms of visceral leishmaniasis at 6 months follow up. Initial cure at the end of therapy was evaluated by repeat spleen or bone marrow aspiration. Patients with initial parasitologic cure were followed for 6 months; patients without absence of clinical signs and symptoms of visceral leishmaniasis were to be evaluated with repeat spleen or bone marrow aspiration to determine final cure.
Two hundred and ninety nine (299) patients received IMPAVIDO and 99 patients received amphotericin B. Approximately, 70% of patients in each arm had previously failed treatment with pentavalent antimony. Initial cure was achieved in 98% of patients in each treatment arm. At 6 months after therapy, 88 (29.5%) IMPAVIDO recipients and 12 (12.1%) amphotericin B recipients continued to have signs and symptoms suggestive of visceral leishmaniasis. These signs or symptoms were attributed to alternative diagnosis in 73 patients. The remaining 27 patients, all in the IMPAVIDO arm, underwent evaluation with splenic or bone marrow aspiration, and 9 (3.0%) were positive for Leishmania amastigotes, indicating relapse. The final cure rates for IMPAVIDO and amphotericin B were 94% and 97%, respectively.
Table 8: Efficacy of IMPAVIDO in Visceral Leishmaniasis in Patients ≥12 years of Age in India - *
- The 95% exact confidence interval for the difference (IV Amphotericin B – IMPAVIDO) in final cure is (-3.0%, 6.8%).
IMPAVIDO
N = 299Amphotericin B Deoxycholate
N = 99End of therapy
Initial Cure
293 (98%)
97 (98%)
6 months after therapy
Final Cure*
282 (94%)
96 (97%)
Treatment Failure
9 (3%)
0 (0)
Not Assessable
8 (3%)
3 (3%)
14.2 Treatment of Cutaneous Leishmaniasis
A placebo controlled study was performed in Colombia where L. panamensis and L. braziliensis are epidemiologically known to be the prevalent infecting Leishmania species, and in Guatemala where L. braziliensis is epidemiologically known to be the prevalent infecting species. The study included male and female patients older than 12 years of age who had newly diagnosed or relapsing cutaneous leishmaniasis without mucosal involvement, parasitologically confirmed, presenting with at least one skin ulcer with minimum area of 50 mm2. Exclusion criteria were AST or ALT ≥2 times upper limit of normal range, bilirubin ≥1.5 times upper limit of normal range, and serum creatinine or BUN >1.5 times upper limit of normal range. Women of reproductive potential were required to use effective contraception for the duration of therapy and for 2 months post therapy.
Patients were randomized to receive IMPAVIDO or placebo in a 2:1 allocation. Patients who weighed less 45 kg received IMPAVIDO 50 mg capsule twice a day, and patients who weighed greater or equal to 45 kg received IMPAVIDO 50 mg capsule three times a day. No patient weighed more than 84 kg. Definite cure was defined as apparent (complete epithelialization of all lesions) or partial cure (incomplete epithelialization, no enlargement by > 50% in lesions, no appearance of new lesions, and negative parasitology if done) at 2 weeks after end of therapy and complete epithelialization of all ulcers at 6 months after end of therapy. The definite cure rate for IMPAVIDO was statistically significantly higher than the cure rate for placebo.
Table 9: Efficacy of IMPAVIDO Compared to Placebo in the Treatment of Cutaneous Leishmaniasis in Colombia and Guatemala - *
- The difference (95% CI) between groups is 36.8% (20.1%, 53.4%) with P-value<0.0001.
IMPAVIDO
Placebo
Definite Cure*
59/89 (66%)
13/44 (30%)
Colombia
40/49 (82%)
9/24 (38%)
Guatemala
19/40 (48%)
4/20 (20%)
An additional study of IMPAVIDO was conducted in Bahia and Manaus, two regions in Brazil where respectively L. braziliensis and L. guyanensis are epidemiologically the prevalent infecting pathogens. Adolescent/adult patients aged 12-65 years received IMPAVIDO orally for 28 days. IMPAVIDO target dose was 2.5 mg/kg/day: patients weighing 15-29 kg received 50 mg once daily, patients weighing 30-45 kg received 50 twice mg daily and patients weighing greater than 46 kg received 50 mg three times daily. The efficacy criteria were initial cure (complete re-epithelialization of the ulcer at 2 months after the end of therapy) followed by definite cure (complete re-epithelialization at 6 months after the end of therapy). Definite cure rate in patients aged 12 years and older was 27/40 (67.5%) for Manaus, Brazil and 34/40 (85%) for Bahia, Brazil.
14.3 Treatment of Mucosal Leishmaniasis
A single arm study was conducted to evaluate the efficacy of IMPAVIDO capsules for the treatment of mucosal leishmaniasis. The study was conducted in Bolivia where L. braziliensis is epidemiologically the prevalent species.
Seventy-nine (79) patients greater than or equal to 18 years of age with a cutaneous leishmaniasis scar plus parasites observed or cultured from lesion material or a positive skin test, and no clinically significant concomitant disease received miltefosine at a target dose of 2.5 mg/kg/day for 28 days. By 12 months after the end of therapy, 49 of the patients (62%) had complete resolution of edema, erythema, infiltration and erosion from the involved mucosal sites.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Each IMPAVIDO capsule contains 50 mg miltefosine in an opaque, red, hard gelatin capsule. IMPAVIDO capsules are supplied in a folded peel/push-through child-resistant blister card. Each blister card contains 14 capsules. Each carton contains two blister cards (NDC 69051-300-01).
Store at 20-25 °C (68-77 °F); excursions permitted to 15-30 °C (59-86 °F). [See USP Controlled Room Temperature]. Protect from moisture.
Dispense only in the original carton.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide)
Dosing Instructions
- •
- IMPAVIDO is administered with food to ameliorate gastrointestinal side effects.
- •
- Instruct the patient to swallow the capsule whole and not to chew it or break it apart. Instruct the patient to complete the full course of therapy.
- •
- Inform the patient that abdominal pain, nausea, vomiting, and diarrhea are common side effects of therapy with IMPAVIDO and instruct the patient to inform their healthcare provider if these gastrointestinal side effects are severe or persistent. Instruct the patient to consume sufficient fluids to avoid dehydration and, consequently, the risk of kidney injury.
Embryo-fetal Toxicity
- •
- Advise pregnant women and females of reproductive potential that IMPAVIDO may cause fetal harm. Advise females to inform their healthcare provider of a known of suspected pregnancy [see Boxed Warning, Contraindications (4), Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
- •
- There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to IMPAVIDO during pregnancy [see Use in Specific Populations (8.1)].
Lactation
- •
- Advise women not to breastfeed during treatment with IMPAVIDO and for 5 months after the last dose [see Use in Specific Populations (8.2)].
Females and Males of Reproductive Potential
- •
- Advise women of reproductive potential to use effective contraception during treatment with IMPAVIDO and for 5 months after the last dose [see Boxed Warning, and Use in Specific Populations (8.1, 8.3)].
- •
- Advise women who use oral contraceptives to use an additional non-oral method of effective contraception during IMPAVIDO therapy, if vomiting and/or diarrhea occurs [see Warnings and Precautions (5.7) and Use in Specific Populations (8.3)].
- •
- Advise women who become pregnant while being treated with IMPAVIDO, to discontinue treatment with IMPAVIDO and seek counseling from their healthcare provider about the potential risk to the fetus [see Boxed Warning and Use in Specific Populations (8.1)].
- •
- Advise male patients that semen quality and sperm parameters may be adversely affected by treatment with IMPAVIDO. Advise male patients that the effects of IMPAVIDO on spermatogenesis may persist for an unknown duration of time [see Warnings and Precautions (5.2) and Use in Specific Populations (8.3)].
- •
- Advise females and males of reproductive potential that IMPAVIDO may impair fertility. [see Warnings and Precautions (5.2, 5.3), Use in Specific Populations (8.3), and Nonclinical Toxicology (13.1)].
- •
- Advise male patients that diminution in ejaculate volume, including temporary absence of ejaculate, and scrotal tenderness may occur during treatment with IMPAVIDO. Male patients should report any concerning genitourinary symptoms to their healthcare provider [see Warnings and Precautions (5.2), Adverse Reactions (6.2), and Use in Specific Populations (8.3)].
Distributed by:
Profounda, Inc.
10501 S Orange Ave, STE 124
Orlando, FL 32824
USAPR033-04
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised 3/2025 MEDICATION GUIDE
IMPAVIDO® (Im-PA-vee-do)
(miltefosine)
capsules, for oral useRead this Medication Guide before you start taking IMPAVIDO and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. What is the most important information I should know about IMPAVIDO?
IMPAVIDO may cause serious risks to pregnancy:- Do not take IMPAVIDO if you are pregnant. If you take IMPAVIDO during pregnancy, your baby is at risk for death or serious birth defects. If you become pregnant while taking IMPAVIDO, stop taking IMPAVIDO and talk to your healthcare provider right away if you become pregnant during treatment with IMPAVIDO.
- You should have a pregnancy test before you start taking IMPAVIDO.
- Women who can become pregnant should use effective birth control (contraception) during IMPAVIDO treatment and for 5 months after their last dose of IMPAVIDO. Talk to your healthcare provider about which birth control method is right for you.
- Pregnancy Registry: There is a registry for women who become pregnant during treatment with IMPAVIDO. If you become pregnant or think you may be pregnant while taking IMPAVIDO, tell your healthcare provider right away. Talk to your healthcare provider about registering with the IMPAVIDO Pregnancy Registry. The purpose of this registry is to collect information about your health and your baby’s health. Your healthcare provider can enroll you in this registry by calling 1-866-588-5405.
What is IMPAVIDO?
IMPAVIDO is a prescription medicine used to treat certain types of leishmaniasis in adults and children 12 years of age and older weighing 66 pounds or more with:- visceral leishmaniasis (affecting your internal organs) caused by Leishmania donovani
- cutaneous leishmaniasis (affecting the skin) caused by Leishmania braziliensis, Leishmania guyanensis, and Leishmania panamensis
- mucosal leishmaniasis (affecting the nose, mouth and throat) caused by Leishmania braziliensis
It is not known if IMPAVIDO is safe and effective in other types of leishmaniasis.
It is not known if IMPAVIDO is safe and effective in children under 12 years of age.Do not take IMPAVIDO if you: - are pregnant, plan to become pregnant, or become pregnant during treatment with IMPAVIDO. See “What is the most important information I should know about IMPAVIDO?”
- have Sjögren-Larsson syndrome
- are allergic to miltefosine or any of the ingredients in IMPAVIDO. See the end of this Medication Guide for a complete list of the ingredients in IMPAVIDO.
Before taking IMPAVIDO, tell your healthcare provider about all your medical conditions, including if you: - have or have had eye problems.
- have kidney or liver problems. Your healthcare provider should do blood tests to check your kidneys and liver before you start, during and after your treatment with IMPAVIDO.
- are breastfeeding or plan to breastfeed. It is not known if IMPAVIDO passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take IMPAVIDO. You should not breastfeed while you take IMPAVIDO and for 5 months after your last dose of IMPAVIDO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take IMPAVIDO? - Take IMPAVIDO exactly as your healthcare provider tells you to.
- Complete your full 28-day IMPAVIDO treatment.
- Take IMPAVIDO capsules whole. Do not break, crush, dissolve, or chew IMPAVIDO before swallowing.
- Take IMPAVIDO with food to help reduce stomach problems.
What are the possible side effects of IMPAVIDO?
IMPAVIDO may cause serious side effects, including:
See “What is the most important information I should know about IMPAVIDO?”-
possible fertility problems. When used in both male humans and animals, IMPAVIDO decreased semen quality, sperm count and semen volume (the amount of fluid released upon ejaculation). IMPAVIDO may cause fertility problems in males. It is not known how long these problems may last. IMPAVIDO may also cause testicular pain and absence of ejaculate.
When used in female animals, IMPAVIDO caused abnormal changes to their menstrual cycles. IMPAVIDO may cause fertility problems in females.
Talk to your healthcare provider if fertility problems are a concern for you. - decreased effectiveness of oral contraceptive pills. If you take birth control pills, vomiting, diarrhea or both may cause your birth control pills to be less effective at preventing pregnancy. If you have vomiting, diarrhea, or both together while taking IMPAVIDO, use an additional non-oral method of birth control, such as male condoms with spermicide, until you are no longer having vomiting or diarrhea, but do not stop taking IMPAVIDO. Talk with your healthcare provider if you have questions about birth control methods that may be right for you during this time.
- kidney problems. Your healthcare provider will do tests every week and for 4 weeks after your treatment with IMPAVIDO has ended to check your kidneys.
- liver problems. Your healthcare provider will do tests to check your liver while you are taking IMPAVIDO.
- stomach problems. IMPAVIDO can cause vomiting, diarrhea, and dehydration. Call your healthcare provider right away if you have severe vomiting and diarrhea that does not go away. Drink a lot of fluids to help prevent dehydration or kidney problems if you are having vomiting and diarrhea.
- decreased platelet count (thrombocytopenia). Some people who were treated for visceral leishmaniasis had decreased platelets. If you have visceral leishmaniasis, your healthcare provider will check your platelet count while you are taking IMPAVIDO. Tell your healthcare provider if you have unusual bleeding or bruising during treatment with IMPAVIDO. This could be a sign of decreased platelet counts, which may reduce the ability of your blood to clot.
- severe skin reactions. IMPAVIDO can cause a rare but serious skin reaction called Stevens-Johnson Syndrome. This may need to be treated in a hospital and may be life-threatening. If you develop a skin rash with blisters, peeling rash, sores in the mouth, hives or any other allergic reactions while taking IMPAVIDO, stop taking IMPAVIDO. Call your healthcare provider right away or get emergency help.
- eye problems. Leishmaniasis infection can cause eye problems. In addition, taking IMPAVIDO can cause eye problems such as inflammation of the cornea (keratitis), which is the clear tissue that covers the pupil; inflammation of the sclera (scleritis), which is the white part of your eye; and inflammation of the front portion of the eye, the iris, which is the colored part of the eye (anterior uveitis or iritis).
IMPAVIDO can cause, in one or both of your eyes, one or more of the following problems:
- redness
- pain
- increased tearing
- lights hurting your eyes (light sensitivity)
- dim or blurry vision
- a white spot over your eye
- some loss of vision or complete loss of vision (blindness)
You may notice one or more of these eye problems after taking IMPAVIDO for a few days or over many weeks. Before starting IMPAVIDO, your healthcare provider may tell you to see an eye doctor (ophthalmologist) who may examine your eyes before you start taking IMPAVIDO.
If while taking IMPAVIDO, you notice any of the eye problems listed above, stop taking IMPAVIDO right away and call your healthcare provider or an eye doctor (ophthalmologist) right away. If you do not stop taking IMPAVIDO right away, there is a chance that your eyesight can become worse or you can even become blind, which can be permanent.
The most common side effects of IMPAVIDO include:
- nausea
- decreased appetite
- skin itching
- fever
- vomiting
- dizziness
- abnormal liver tests
- tiredness
- diarrhea
- headache
- abnormal kidney tests
- weakness
- stomach pain
- sleepiness
- motion sickness
- enlarged lymph nodes
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of IMPAVIDO. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store IMPAVIDO? - Store IMPAVIDO at room temperature between 68°F to 77°F (20°C to 25°C).
- Protect IMPAVIDO from moisture.
General information about the safe and effective use of IMPAVIDO.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use IMPAVIDO for a condition for which it was not prescribed. Do not give IMPAVIDO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about IMPAVIDO that is written for health professionals.What are the ingredients in IMPAVIDO?
Active ingredient: miltefosine
Inactive ingredients: colloidal silicon dioxide, microcrystalline cellulose, lactose monohydrate, talc, and magnesium stearate. The capsule shell contains gelatin, titanium dioxide, ferric oxide, and purified water.
Distributed by: Profounda, Inc.
PR034-02
For more information, go to www.knighttx.com or call1-844-483-5636. -
PRINCIPAL DISPLAY PANEL - Carton
NDC 69051-300-01 28 Capsules
Impavido®
(miltefosine) capsules
50 mg per CapsuleRx only PROFOUNDA®
Contains 2 blister cards, 14 blisters per card, 1 capsule per blister.
Active ingredient: miltefosine
Inactive ingredients: colloidal silicon dioxide, lactose monohydrate,
magnesium stearate, microcrystalline cellulose, talc. The capsule shell
contains gelatin, titanium dioxide, ferric oxide and purified water.Usual dosage: see enclosed package insert for complete
prescribing information.Keep out of reach of children.
Store at 20-25 °C (68-77 °F); excursions permitted
to 15-30 °C (59-86 °F). [See USP Controlled Room Temperature].Distributed by:
Profounda, Inc.
10501 S Orange Ave, STE 124
Orlando, Florida 32824Impavido®
(miltefosine) capsules
50 mg per CapsuleNDC 69051-300-01
28 Capsules
Lot:
Exp. Date:
-
INGREDIENTS AND APPEARANCE
IMPAVIDO
miltefosine capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69051-300 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MILTEFOSINE (UNII: 53EY29W7EC) (MILTEFOSINE - UNII:53EY29W7EC) MILTEFOSINE 50 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) WATER (UNII: 059QF0KO0R) Product Characteristics Color RED (opaque) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69051-300-01 2 in 1 CARTON 10/29/2015 1 14 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204684 10/29/2015 Labeler - Profounda, Inc. (078862060)