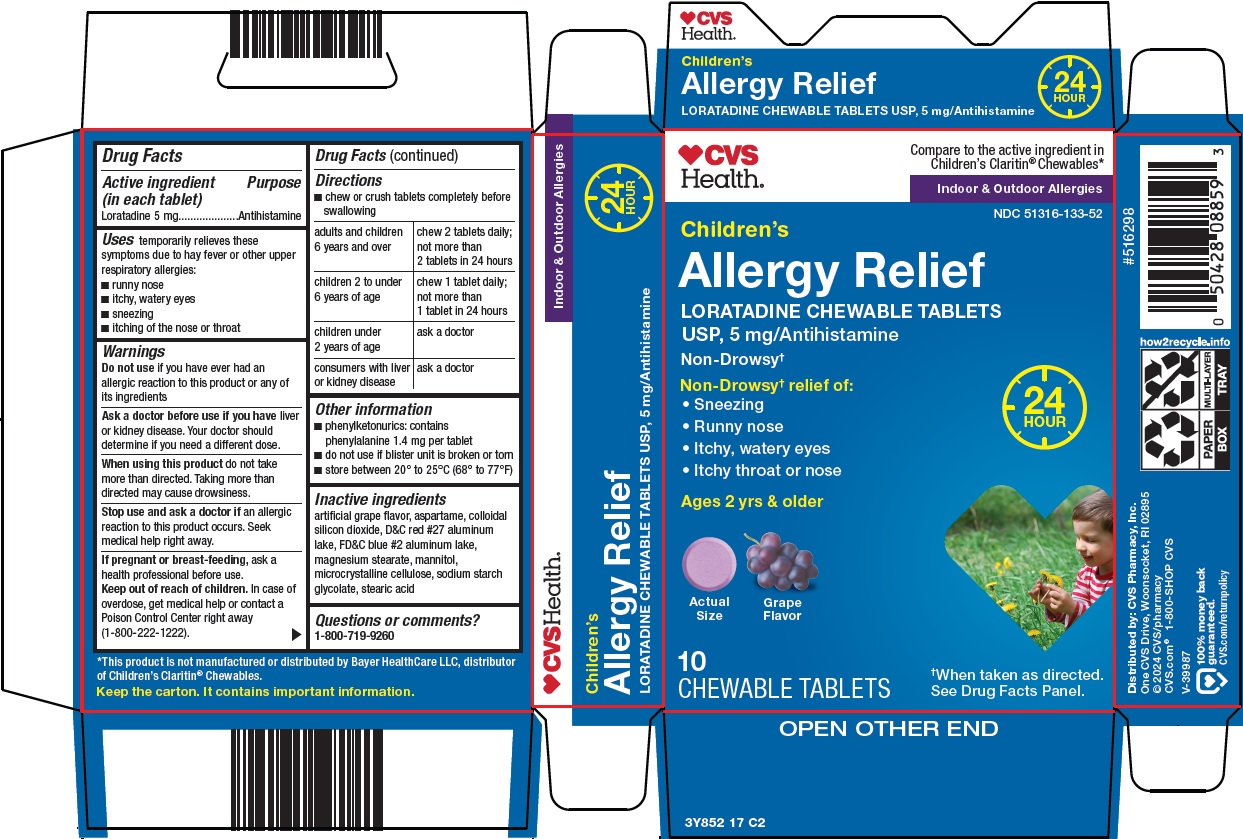
Label: CHILDRENS ALLERGY RELIEF- loratadine tablet, chewable
- NDC Code(s): 51316-133-52
- Packager: CVS WOONSOCKET PRESCRIPTION CENTER, INCORPORATED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
-
Directions
- •
- chew or crush tablets completely before swallowing
adults and children 6 years and over
chew 2 tablets daily; not more than 2 tablets in 24 hours
children 2 to under 6 years of age
chew 1 tablet daily; not more than 1 tablet in 24 hours
children under 2 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
CVS Health®
Compare to the active ingredient in Children’s Claritin® Chewables
Indoor & Outdoor Allergies
Children’s Allergy Relief
LORATADINE CHEWABLE TABLETS USP, 5 mg/Antihistamine
Non-Drowsy†
24 HOUR
Non-Drowsy† relief of:
• Sneezing
• Runny nose
• Itchy, watery eyes
• Itchy throat or nose
Ages 2 yrs & older
Actual Size
Grape Flavor
10 CHEWABLE TABLETS
†When taken as directed.
See Drug Facts Panel.
-
INGREDIENTS AND APPEARANCE
CHILDRENS ALLERGY RELIEF
loratadine tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51316-133 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 5 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color PURPLE (light) Score no score Shape ROUND Size 8mm Flavor GRAPE Imprint Code L3Y8 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51316-133-52 10 in 1 CARTON 06/06/2024 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210033 06/06/2024 Labeler - CVS WOONSOCKET PRESCRIPTION CENTER, INCORPORATED (062312574)