Label: ALLERGY- cetirizine hydrochloride tablet, film coated
- NDC Code(s): 41226-692-14, 41226-692-65, 41226-692-70
- Packager: Kroger Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
adults and children 6 years and over: one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms.
adults 65 years and over: ask a doctor
children under 6 years of age: ask a doctor
consumers with liver or kidney disease: ask a doctor
- OTHER INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
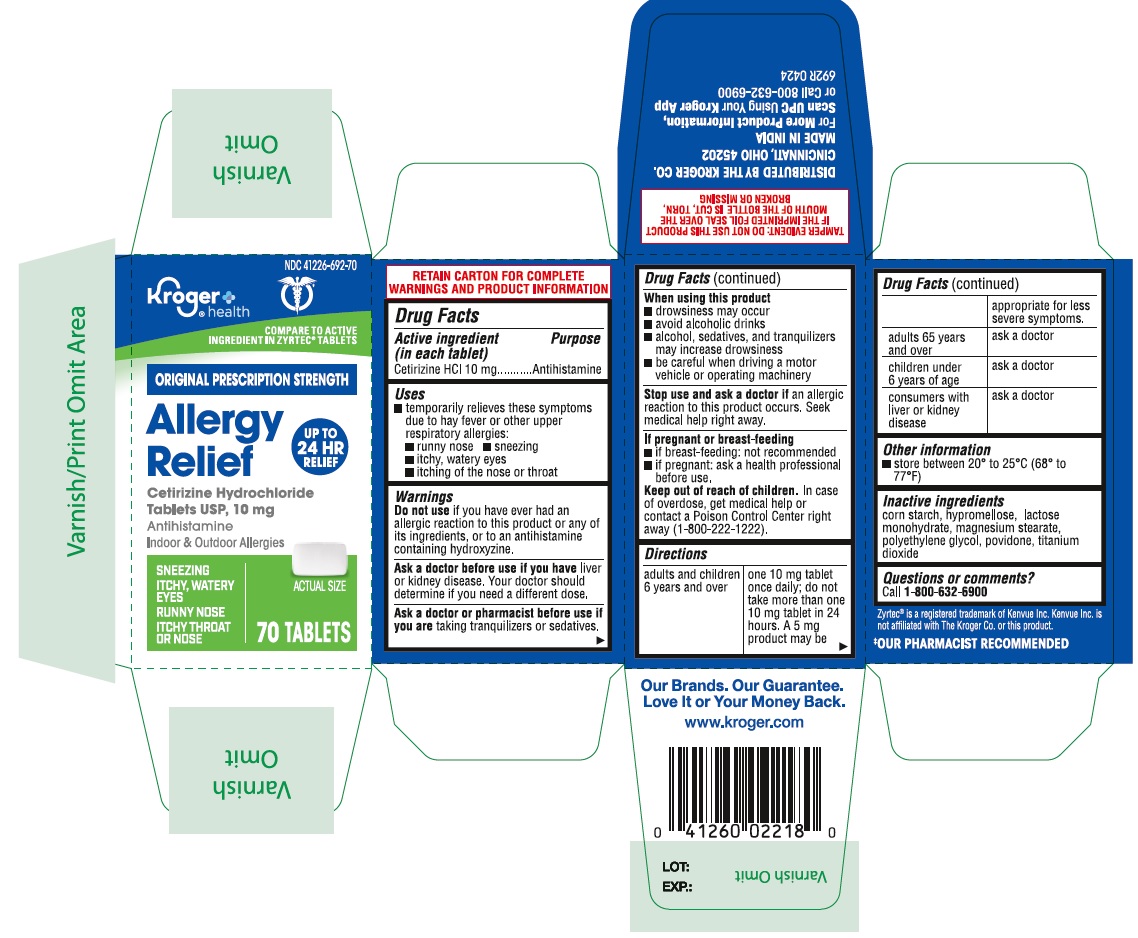
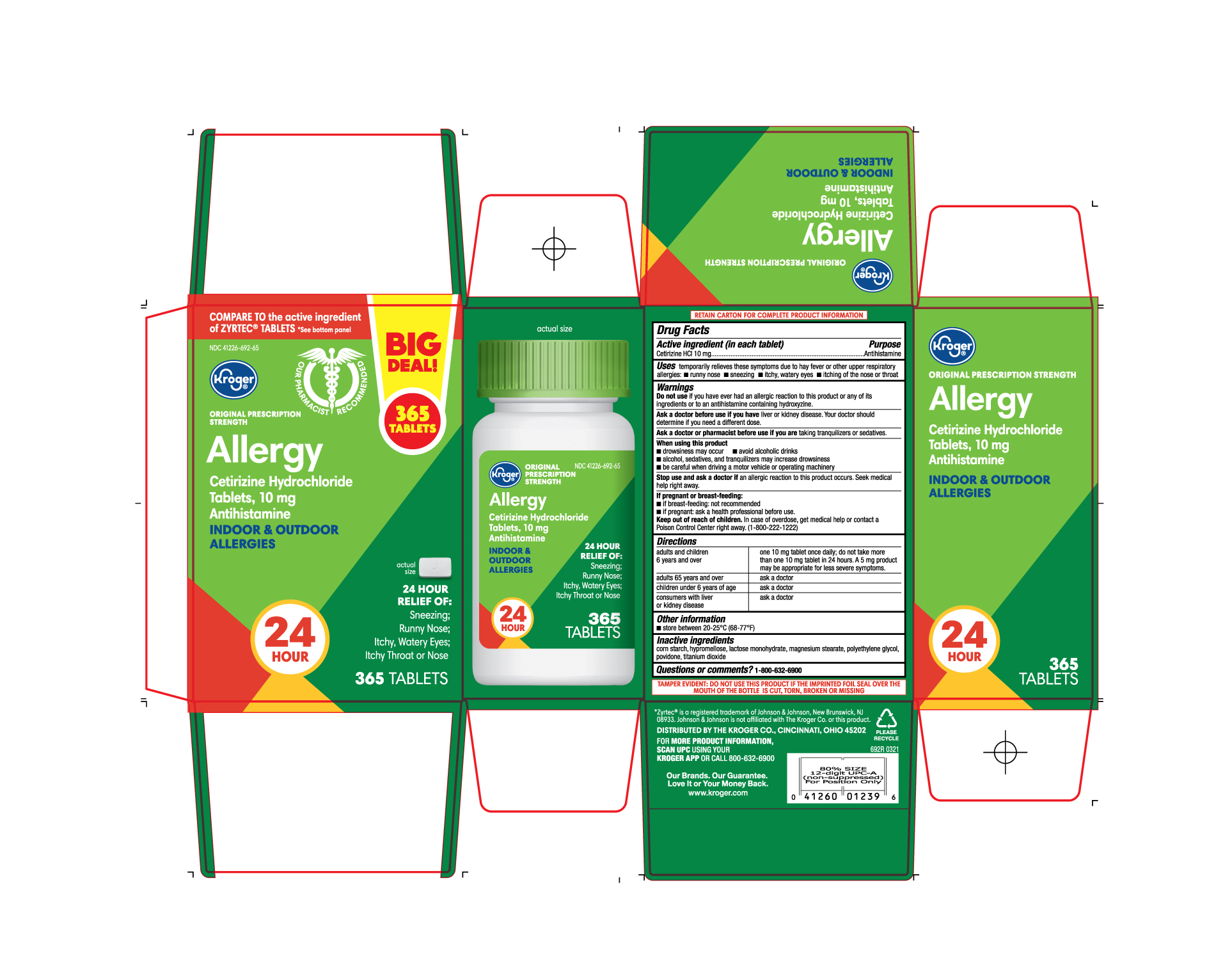
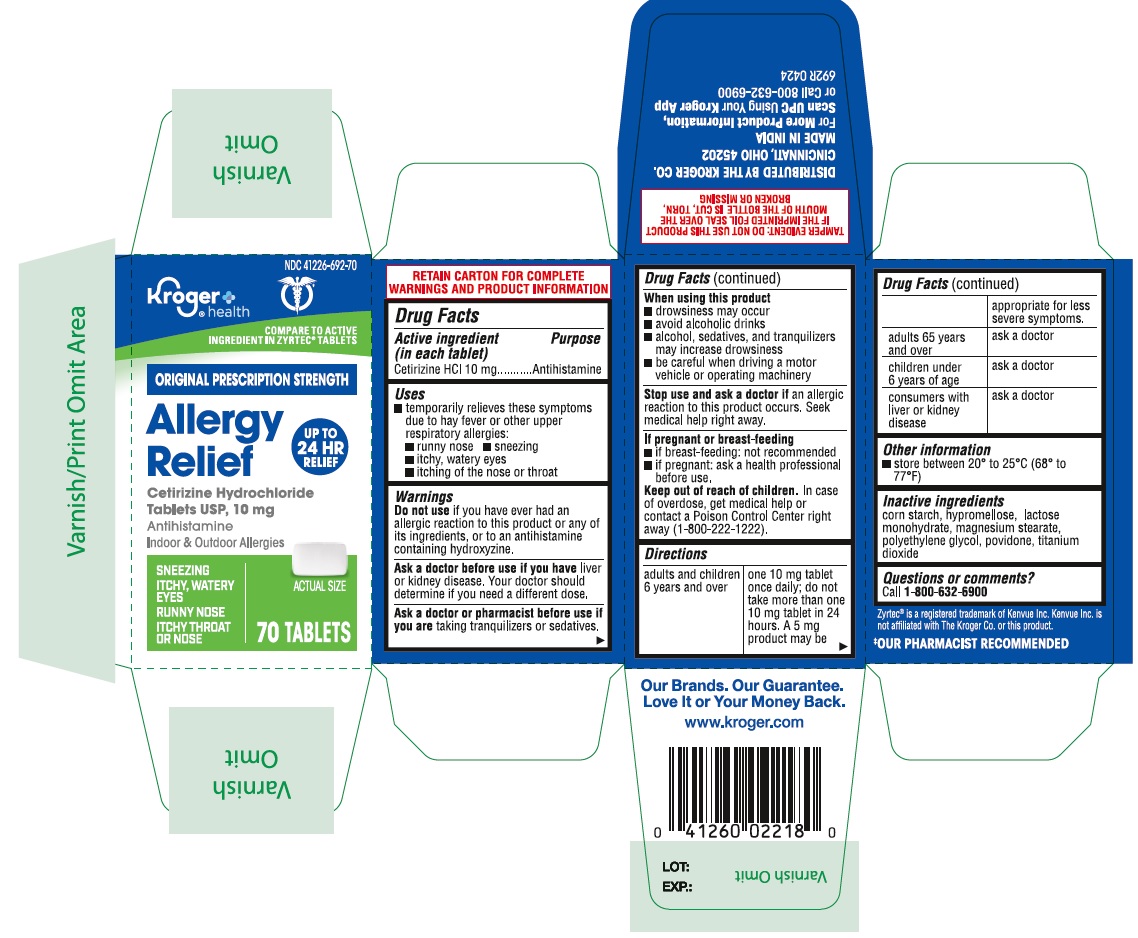
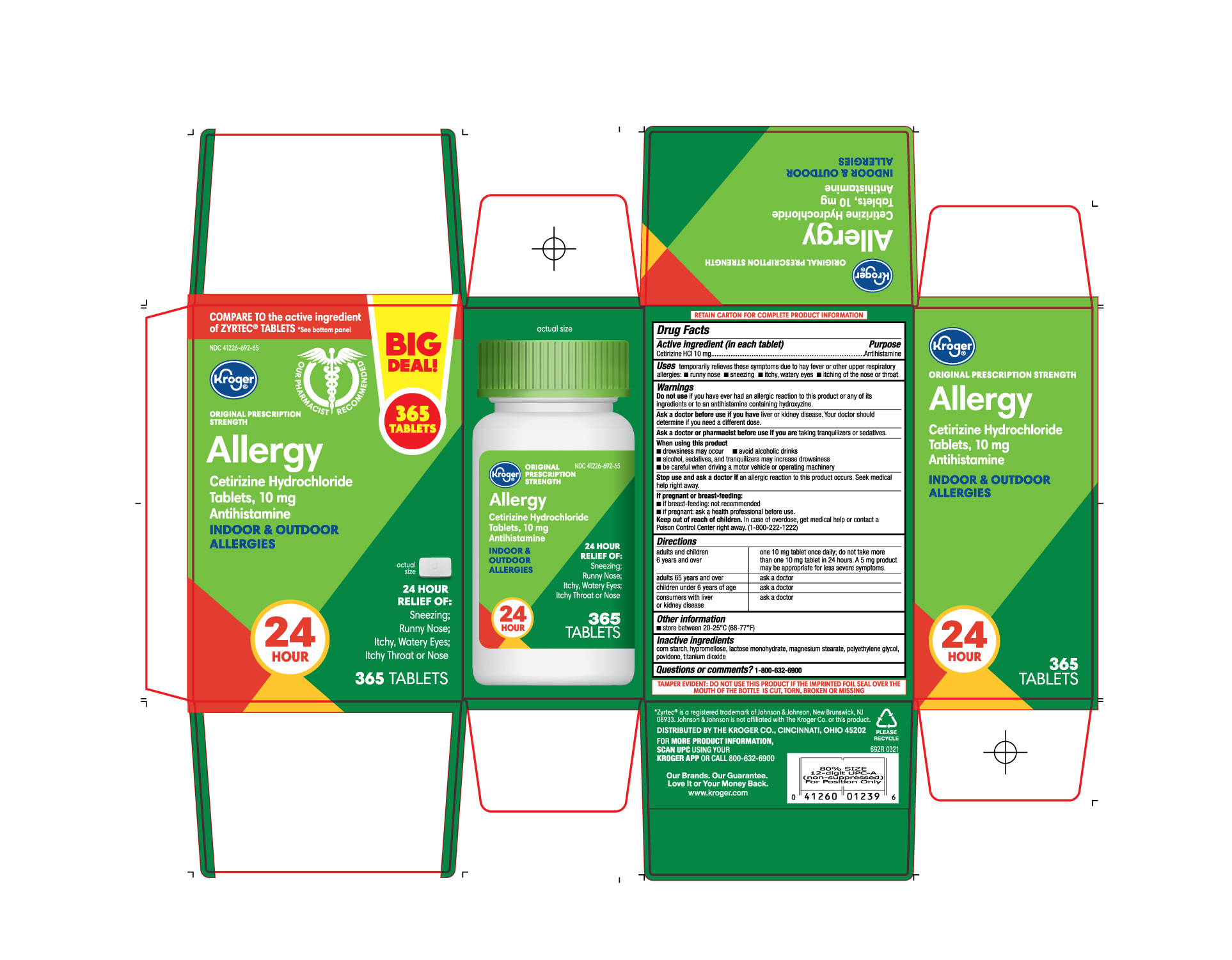
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALLERGY
cetirizine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41226-692 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POVIDONE (UNII: FZ989GH94E) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white ((white to off white)) Score no score Shape RECTANGLE (Rounded-off rectangular shaped) Size 10mm Flavor Imprint Code J;220 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41226-692-65 1 in 1 CARTON 05/11/2021 1 365 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:41226-692-14 2 in 1 CARTON 05/11/2021 2 70 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:41226-692-70 1 in 1 CARTON 06/03/2024 3 70 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078933 05/11/2021 Labeler - Kroger Company (006999528) Registrant - TIME CAP LABORATORIES, INC. (037052099) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LIMITED 925822975 manufacture(41226-692)