Label: LORATADINE AND PSEUDOEPHEDRINE tablet, extended release
- NDC Code(s): 51660-491-15, 51660-491-69
- Packager: Ohm Laboratories Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 15, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS (IN EACH TABLET)
- PURPOSE
-
USES
- •
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- sneezing
- •
- itchy, watery eyes
- •
- runny nose
- •
- itching of the nose or throat
- •
- reduces swelling of nasal passages
- •
- temporarily relieves sinus congestion and pressure
- •
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- •
- temporarily restores freer breathing through the nose
-
WARNINGS
Do not use
- •
- if you have ever had an allergic reaction to this product or any of its ingredients
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- •
- heart disease
- •
- thyroid disease
- •
- high blood pressure
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
- •
- liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed.
Taking more than directed may cause drowsiness.
- DIRECTIONS
- OTHER INFORMATION
-
INACTIVE INGREDIENTS
calcium carbonate, colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose, iron oxide black, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, pregelatinized starch, propylene glycol, shellac glaze, sodium alginate, sodium citrate, talc and titanium dioxide
- QUESTIONS?
-
PACKAGE LABEL. PRINCIPAL DISPLAY PANEL
NDC 51660-491-69
Original Prescription Strength
*Compare to the active ingredients of Claritin-D® 24 Hour
NON-DROWSY**
Allergy Relief & Nasal Decongestant
24 Hour Allergy Relief
Loratadine, USP 10 mg/Antihistamine
Pseudoephedrine Sulfate, USP 240 mg/Nasal Decongestant
Indoor & Outdoor Allergies
Relief of:
- •
- Nasal & Sinus Congestion Due to Colds or Allergies
- •
- Sneezing
- •
- Runny Nose
- •
- Itchy, Watery Eyes
- •
- Itchy Throat or Nose Due to Allergies
Allergy & Congestion 10 Extended-Release Tablets
**When taken as directed. See Drug Facts Panel.
*The product is not manufactured or distributed by Schering-Plough Healthcare Products, Inc. CLARITIN-D® 24 HOUR is a registered trademark of Schering Corporation.
-
Principal Display Panel
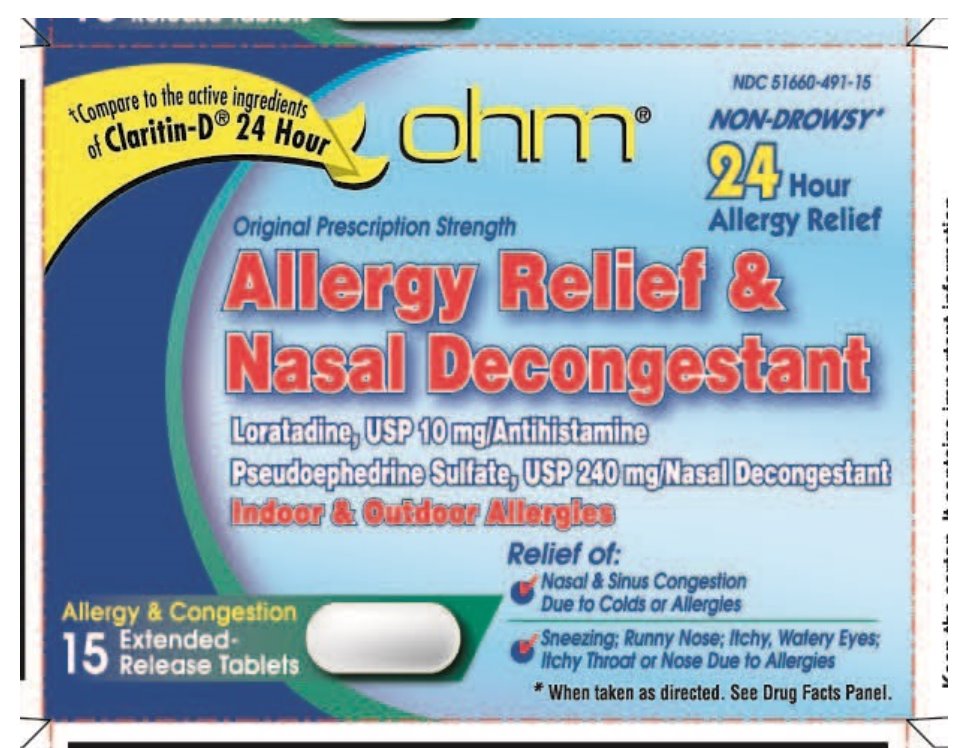
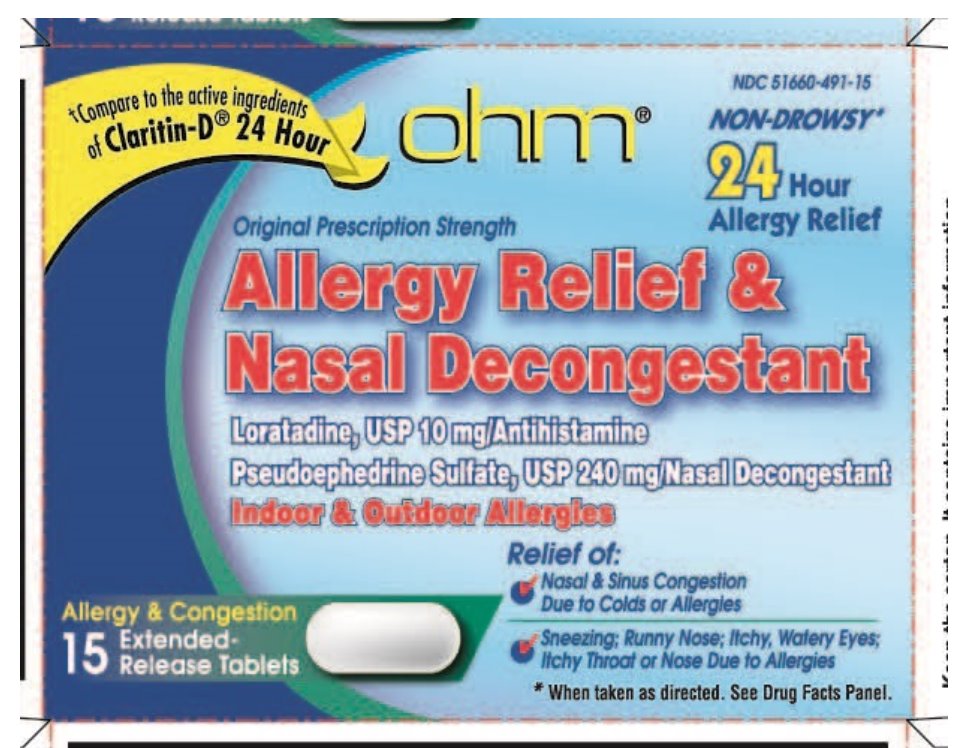
NDC 51660-491-15
Original Prescription Strength
*Compare to the active ingredients of Claritin-D® 24 Hour
NON-DROWSY**
Allergy Relief & Nasal Decongestant
24 Hour Allergy Relief
Loratadine, USP 15 mg/Antihistamine
Pseudoephedrine Sulfate, USP 240 mg/Nasal Decongestant
Indoor & Outdoor Allergies
Relief of:
- •
- Nasal & Sinus Congestion Due to Colds or Allergies
- •
- Sneezing
- •
- Runny Nose
- •
- Itchy, Watery Eyes
- •
- Itchy Throat or Nose Due to Allergies
Allergy & Congestion 15 Extended-Release Tablets
**When taken as directed. See Drug Facts Panel.
*The product is not manufactured or distributed by Schering-Plough Healthcare Products, Inc. CLARITIN-D® 24 HOUR is a registered trademark of Schering Corporation.
-
INGREDIENTS AND APPEARANCE
LORATADINE AND PSEUDOEPHEDRINE
loratadine and pseudoephedrine tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51660-491 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg PSEUDOEPHEDRINE SULFATE (UNII: Y9DL7QPE6B) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE SULFATE 240 mg Inactive Ingredients Ingredient Name Strength CALCIUM CARBONATE (UNII: H0G9379FGK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FERROSOFERRIC OXIDE (UNII: XM0M87F357) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SODIUM ALGINATE (UNII: C269C4G2ZQ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color white Score no score Shape CAPSULE Size 17mm Flavor Imprint Code RX724 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51660-491-69 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 01/15/2020 2 NDC:51660-491-15 15 in 1 BLISTER PACK; Type 0: Not a Combination Product 01/15/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076557 11/17/2004 Labeler - Ohm Laboratories Inc. (184769029) Registrant - Ranbaxy Pharmaceuticals Inc. (937890044) Establishment Name Address ID/FEI Business Operations Ohm Laboratories Inc. 184769029 MANUFACTURE(51660-491)