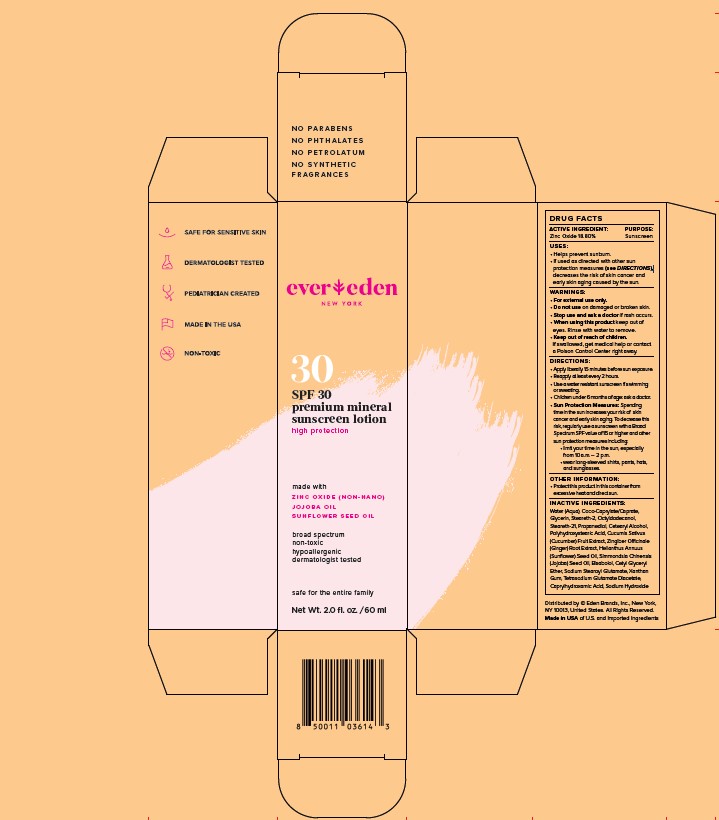
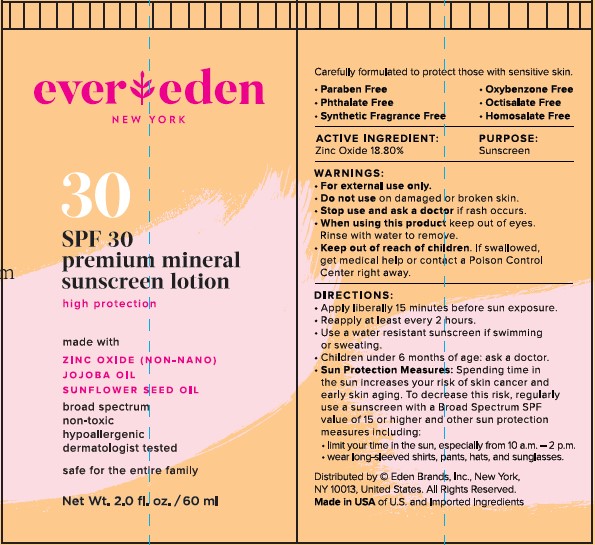
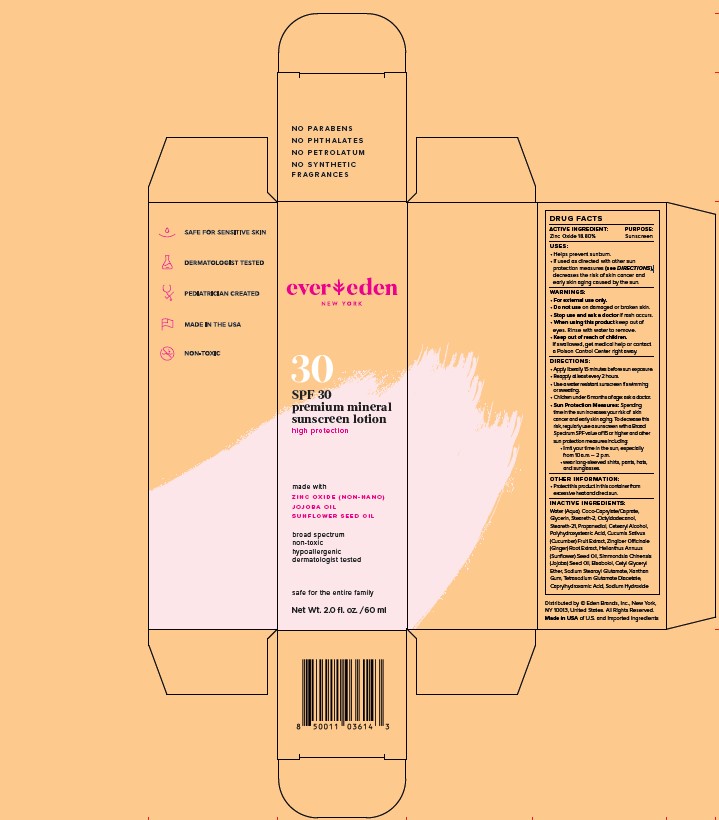
Label: EVEREDEN PREMIUM MINERAL SUNSCREEN SPF 30- zinc oxide lotion
- NDC Code(s): 72113-102-14
- Packager: Eden Brands Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- STATEMENT OF IDENTITY
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
WARNINGS:
- For external use only.
- Do not use on damaged or broken skin.
- Stop use and ask a doctor if rash occurs.
- When using this product keep out of eyes. Rinse with water to remove.
- Keep out of reach of children. if swallowed, get medical help or contact a Poison Control Center right away.
-
INSTRUCTIONS FOR USE
DIRECTIONS:
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Children under 6 months of age: ask a doctor
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
-limit your time in the sun, especially from 10am-2pm
-wear long-sleeved shirts, pants, hats, and sungalsses.
-
INACTIVE INGREDIENT
INACTIVE INGREDIENTS:
Water (Aqua), Coco-Caprylate/Caprate, Glycerin, Steareth-2, Octyldodecanol, Steareth-21, Propanediol, Cetearyl Alcohol, Polyhydroxystearic Acid, Cucumis Sativus (Cucumber) Fruit Extract, Zingiber Officinale (Ginger) Root Extract, Helianthus Annuus (Sunflower) Seed Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Bisabolol, Cetyl Glyceryl Ether, Sodium Stearoyl Glutamate, Xanthan Gum, Tetrasodium Glutamate Diacetate, Caprylhydroxamic Acid, Sodium Hydroxide
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EVEREDEN PREMIUM MINERAL SUNSCREEN SPF 30
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72113-102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 188 mg in 1 g Inactive Ingredients Ingredient Name Strength OCTYLDODECANOL (UNII: 461N1O614Y) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) CUCUMIS SATIVUS WHOLE (UNII: 50560UL2YV) ZINGIBER OFFICINALE WHOLE (UNII: IN6Q3S3414) XANTHAN GUM (UNII: TTV12P4NEE) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) WATER (UNII: 059QF0KO0R) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) PROPANEDIOL (UNII: 5965N8W85T) SODIUM HYDROXIDE (UNII: 55X04QC32I) GLYCERIN (UNII: PDC6A3C0OX) HELIANTHUS ANNUUS WHOLE (UNII: 17S27ZT6KR) SIMMONDSIA CHINENSIS SEED (UNII: D24K2Q1F6H) CETYL GLYCERYL ETHER (UNII: P9FNL3D0MN) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72113-102-14 1 in 1 CARTON 04/19/2021 1 60 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 04/19/2021 Labeler - Eden Brands Inc (081062339)