Label: GOLD BOND MEDICATED MAXIMUM STRENGTH FOOT- menthol powder
- NDC Code(s): 41167-0171-2, 41167-0171-6
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
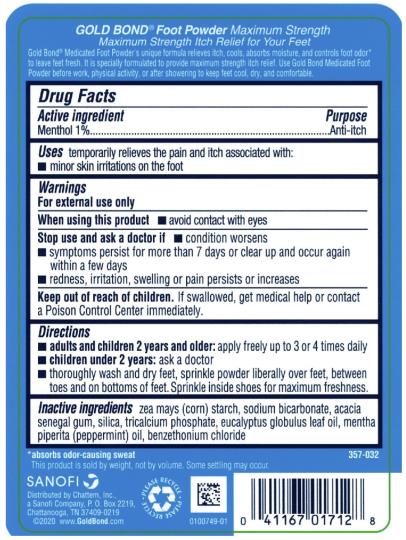
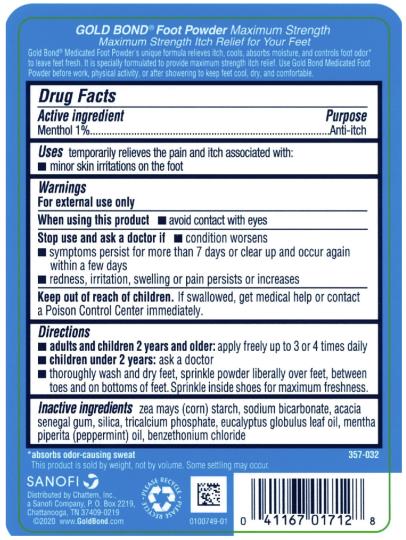
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GOLD BOND MEDICATED MAXIMUM STRENGTH FOOT
menthol powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0171 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 100 g Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) SODIUM BICARBONATE (UNII: 8MDF5V39QO) ACACIA (UNII: 5C5403N26O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRICALCIUM PHOSPHATE (UNII: K4C08XP666) EUCALYPTUS OIL (UNII: 2R04ONI662) PEPPERMINT OIL (UNII: AV092KU4JH) BENZETHONIUM CHLORIDE (UNII: PH41D05744) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0171-2 283 g in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2021 2 NDC:41167-0171-6 113 g in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/01/2021 Labeler - Chattem, Inc. (003336013)