Label: ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (PIMIENTA CALIENTE) - BROWN- octinoxate and zinc oxide lipstick
ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROJO DESEO) - RED- octinoxate and zinc oxide lipstick
ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROJO ESIKA) - RED (octino .......RO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (NUDE MOCCHA) - ORANGE- octinoxate and zinc oxide lipstick
ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (BRONCE METALLIC) - BROWN- octinoxate and zinc oxide lipstick
ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25- octinoxate and zinc oxide kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 13537-958-01, 13537-958-02, 13537-959-03, 13537-959-04, view more13537-960-05, 13537-960-06, 13537-966-07, 13537-966-08, 13537-967-09, 13537-967-10, 13537-968-11, 13537-968-12, 13537-969-13, 13537-969-14, 13537-970-17, 13537-970-18, 13537-971-19, 13537-971-20, 13537-972-21, 13537-972-22, 13537-973-23, 13537-973-24, 13537-974-23, 13537-974-24, 13537-975-23, 13537-975-24, 13537-976-23, 13537-976-24, 13537-977-23, 13537-977-24, 13537-978-23, 13537-978-24, 13537-979-23, 13537-979-24, 13537-980-23, 13537-980-24, 13537-981-23, 13537-981-24, 13537-982-23, 13537-982-24, 13537-983-23, 13537-983-24, 13537-984-23, 13537-984-24, 13537-985-23, 13537-985-24, 13537-986-23, 13537-986-24, 13537-987-27, 13537-987-28 - Packager: Ventura Corporation LTD DUNS
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 26, 2018
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
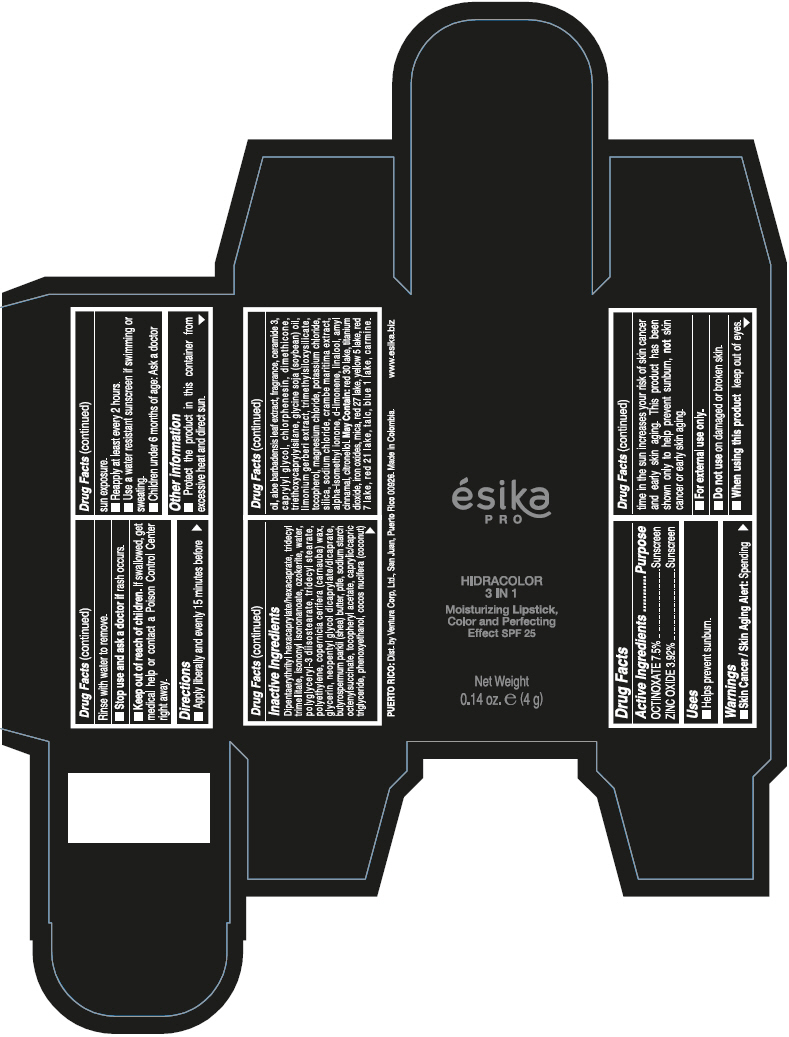
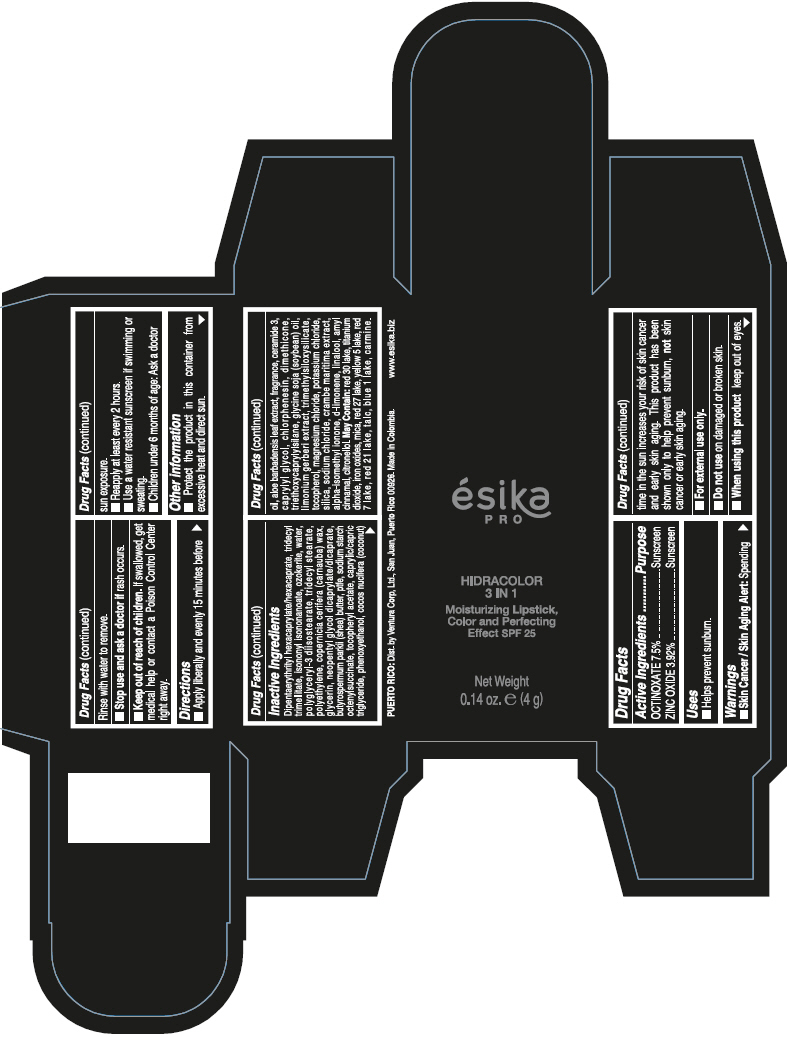
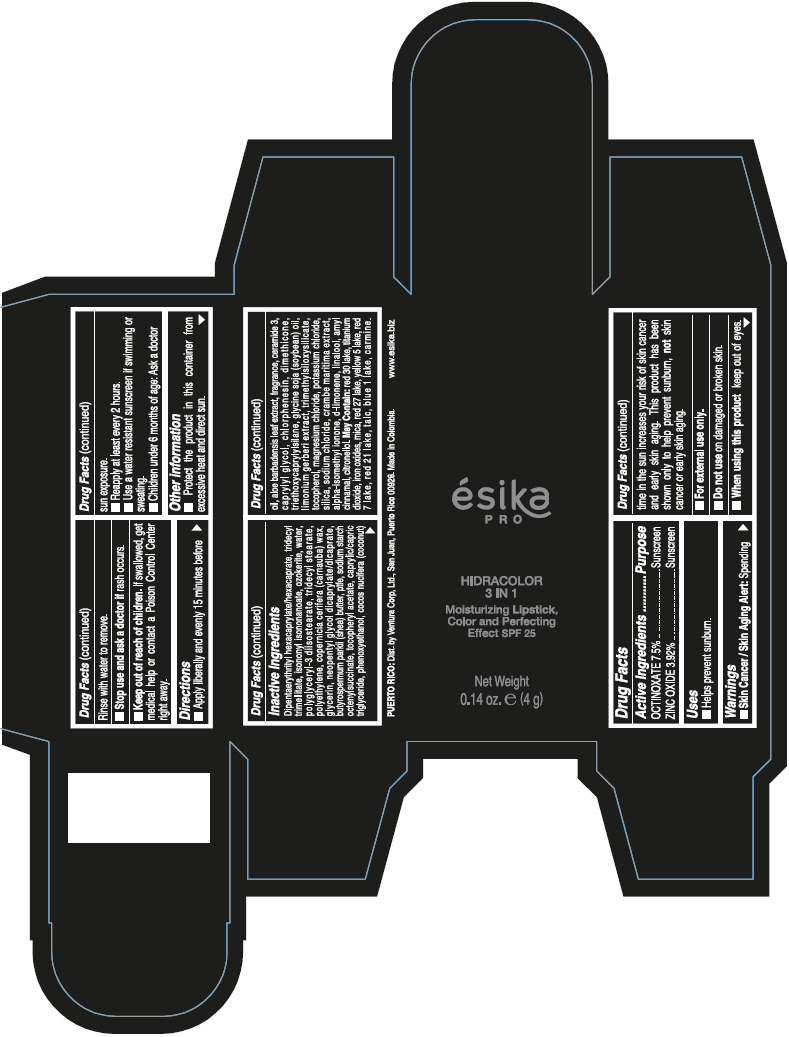
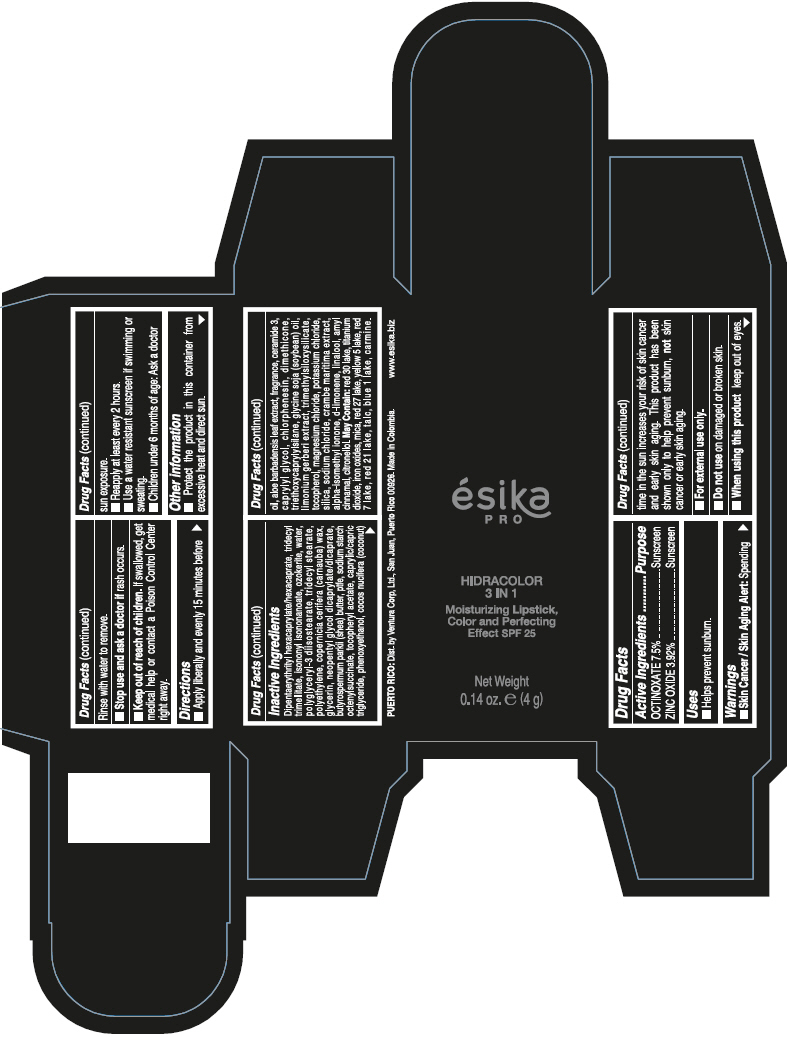
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
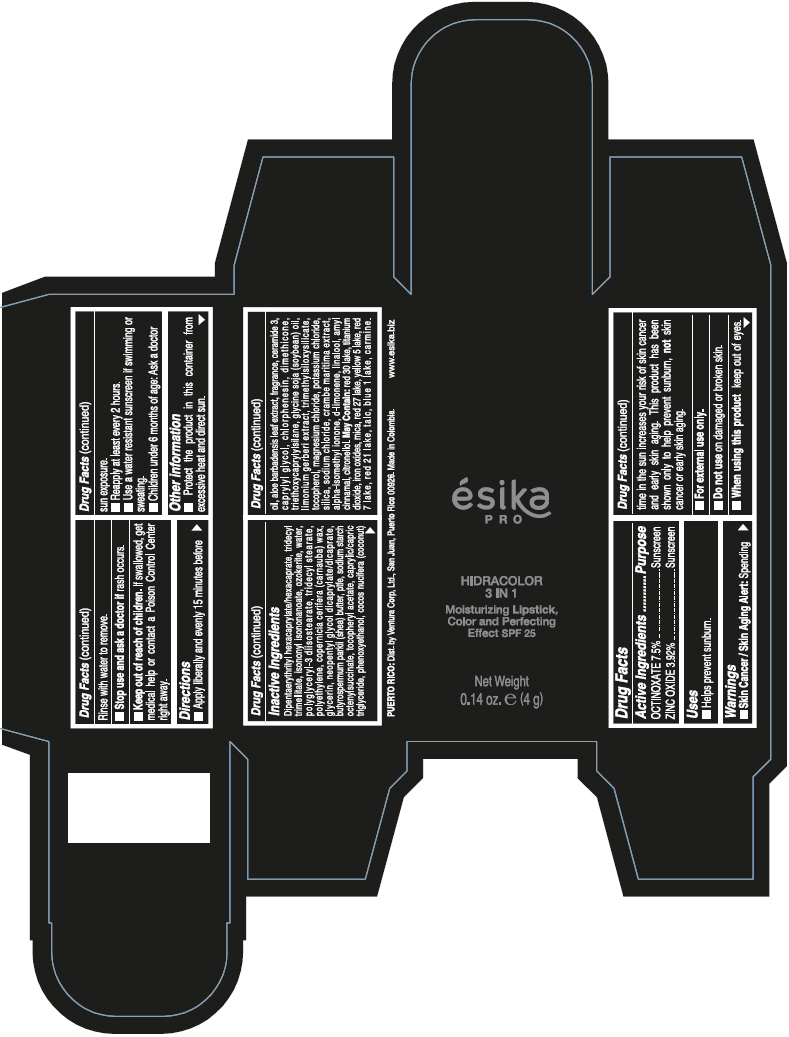
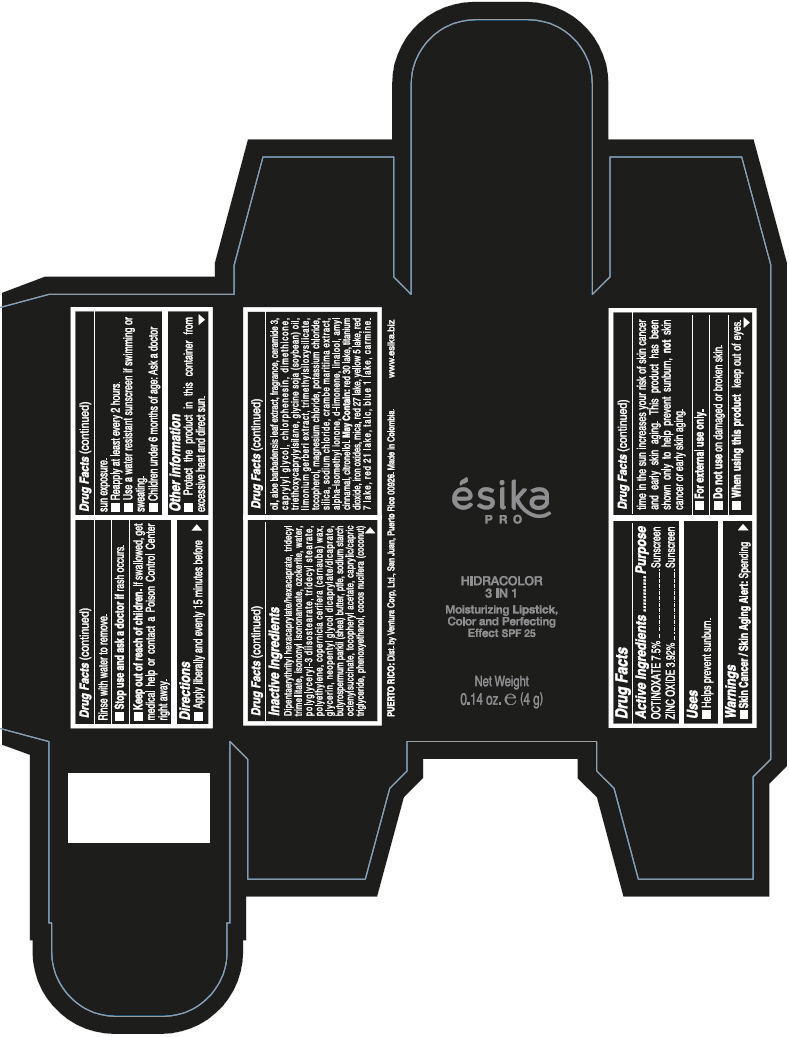
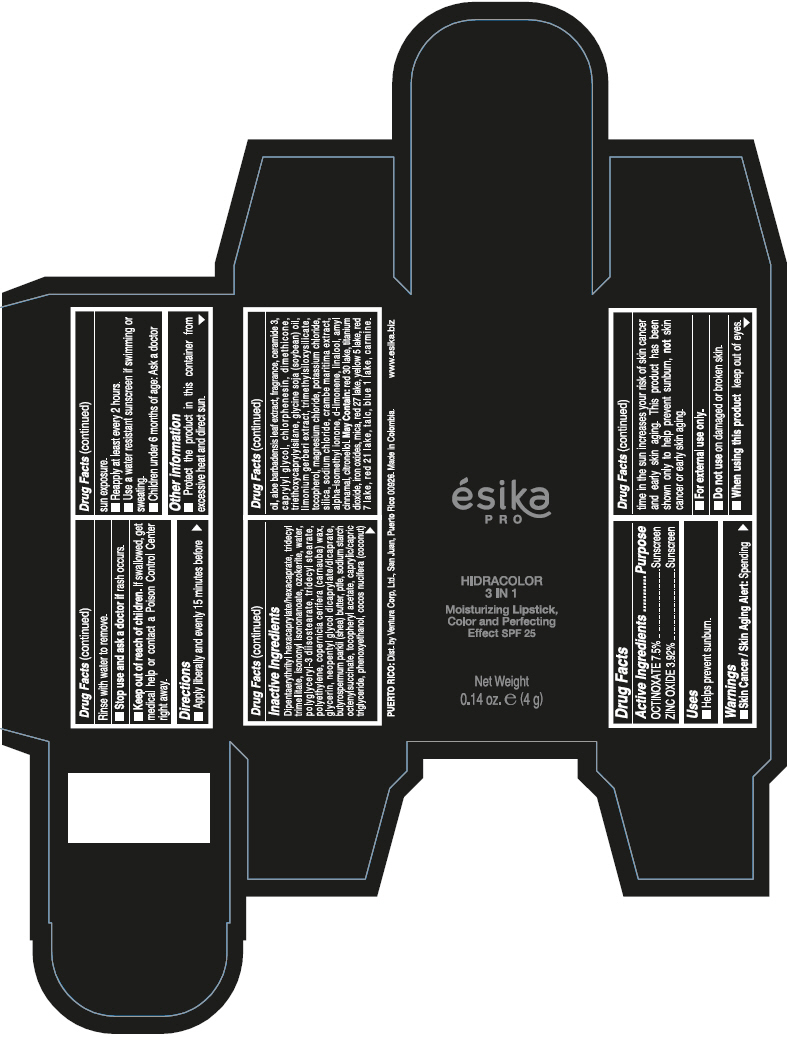
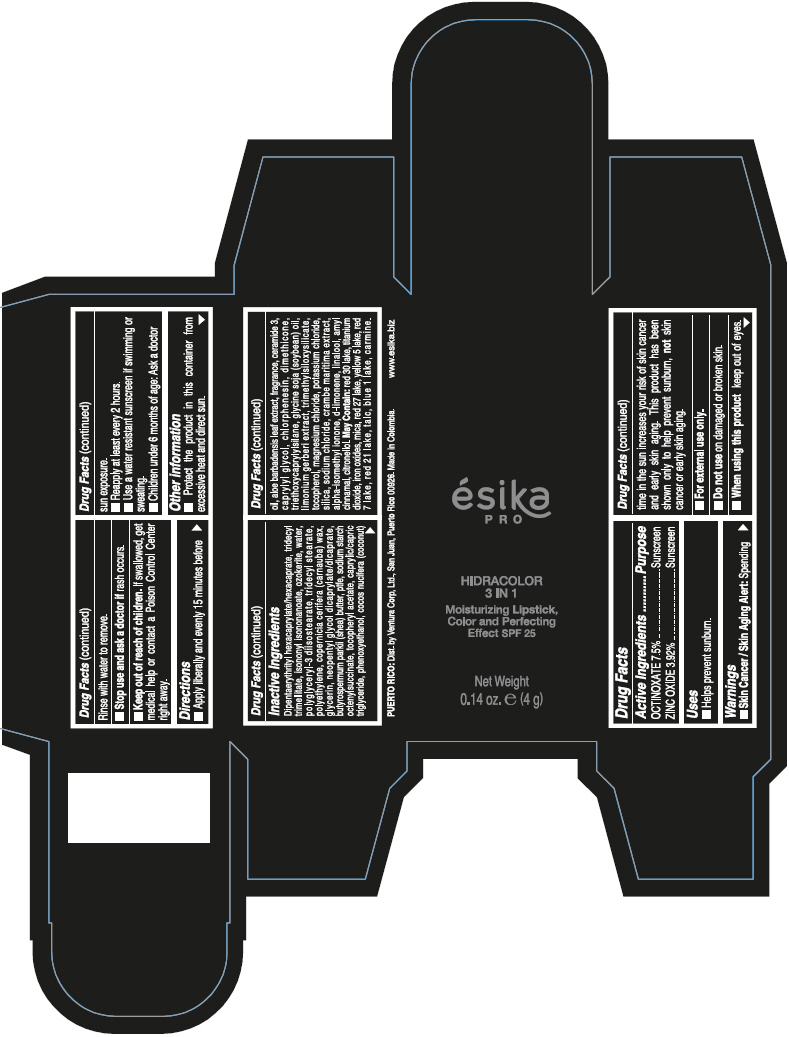
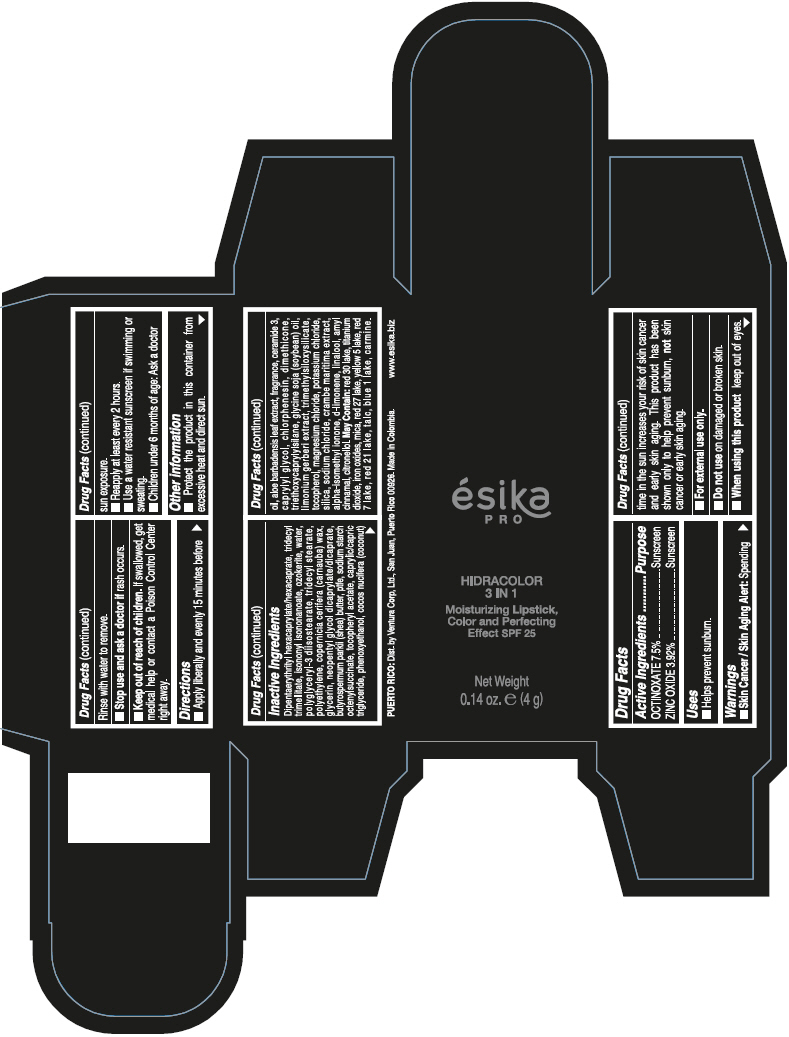
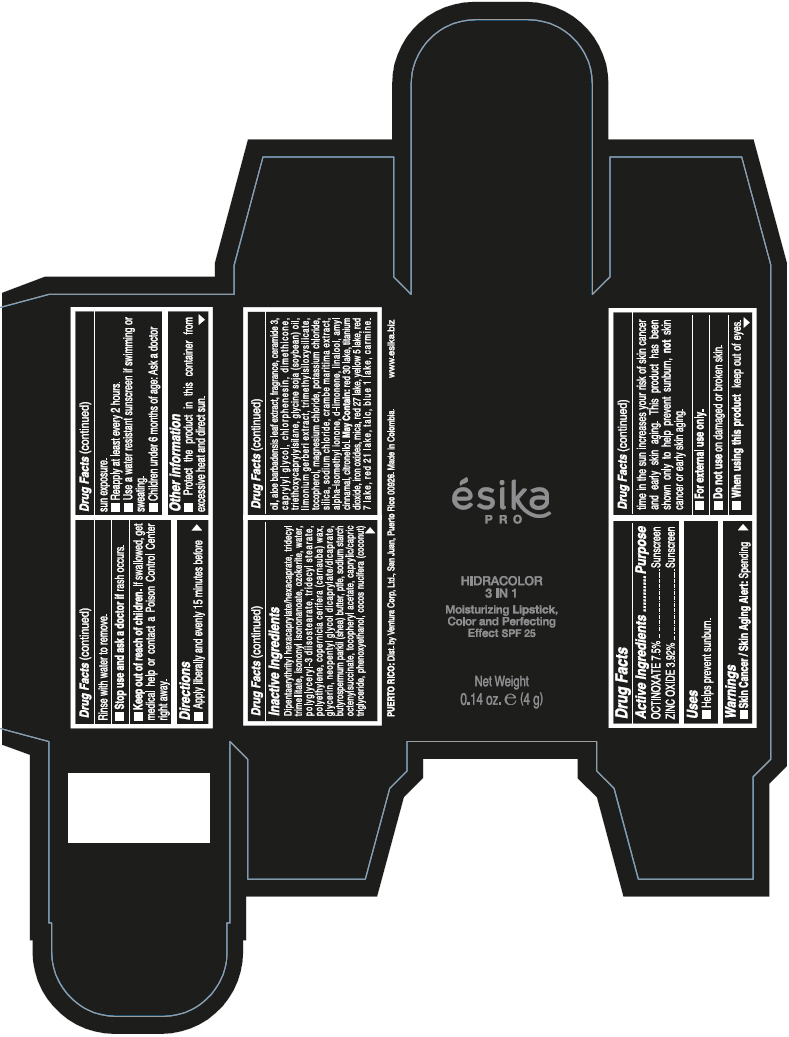
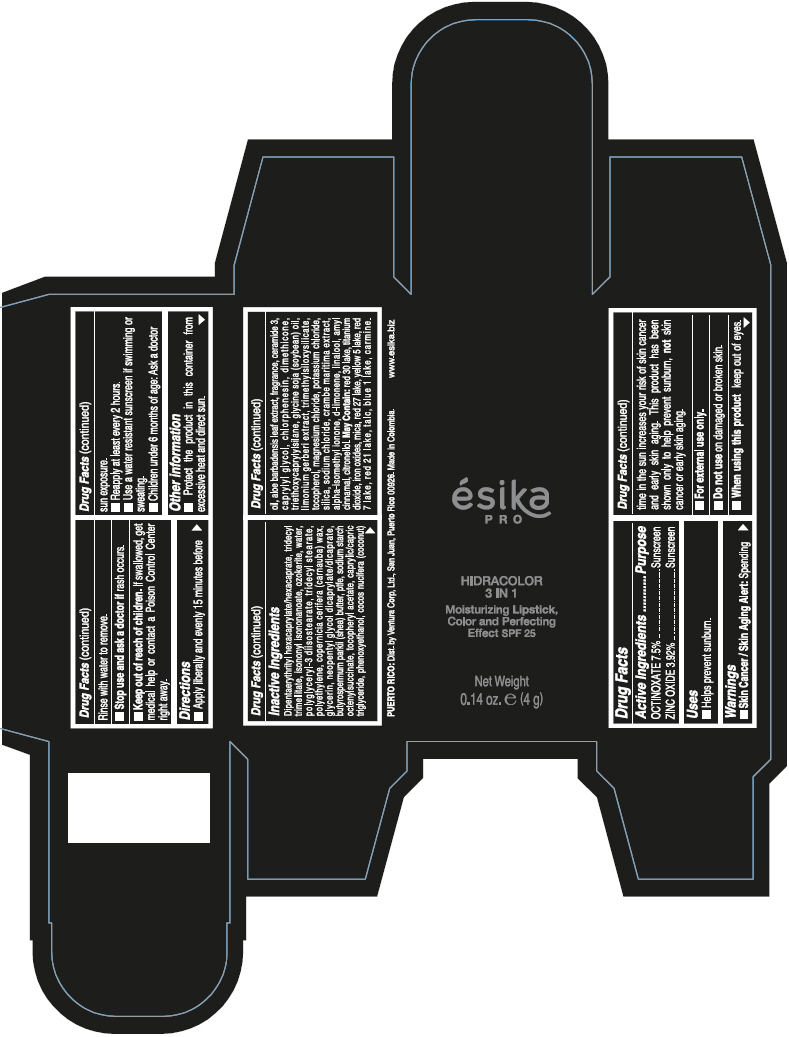
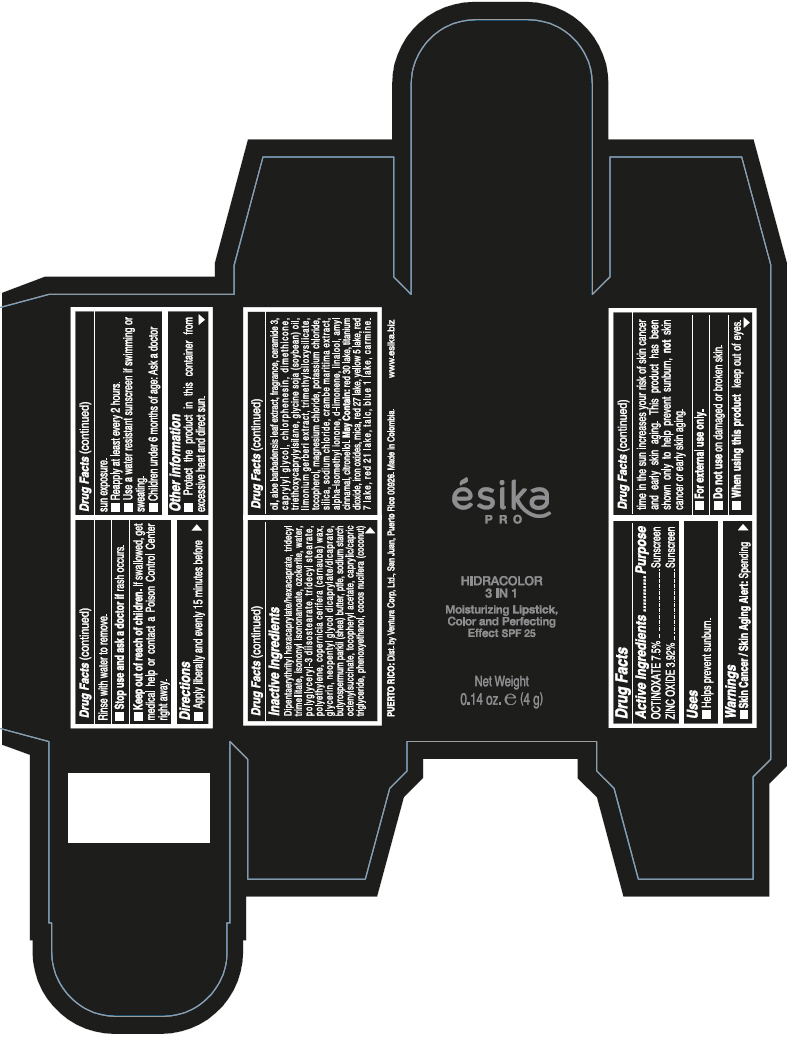
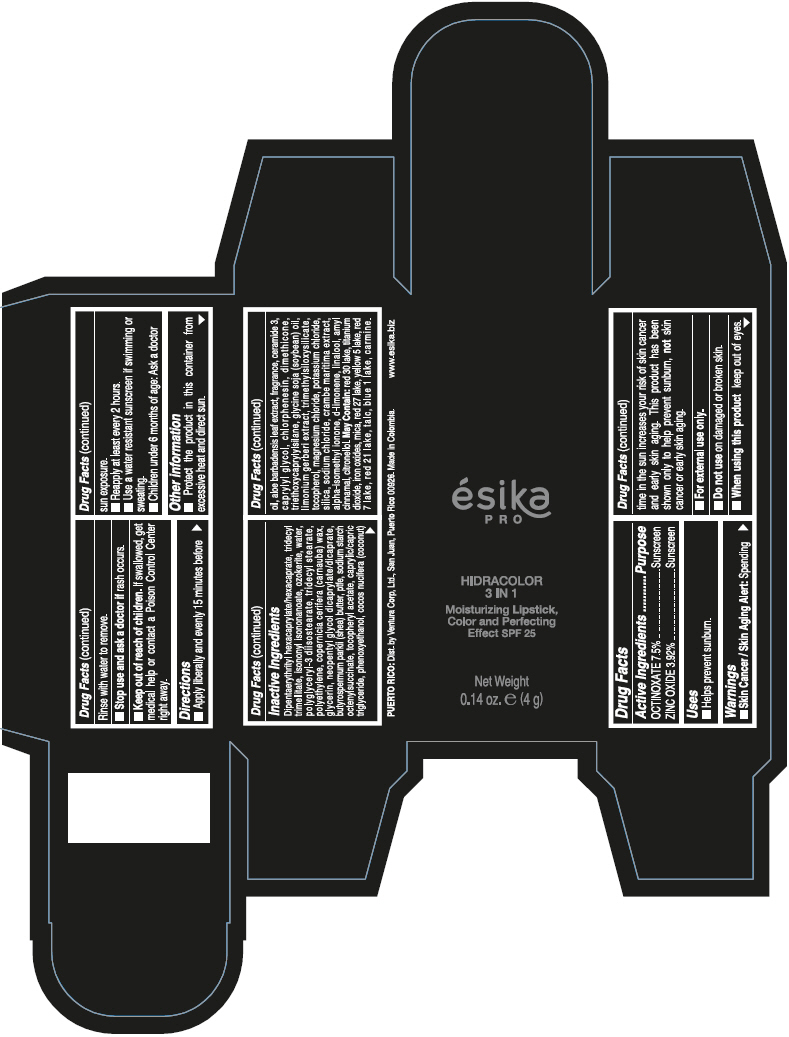
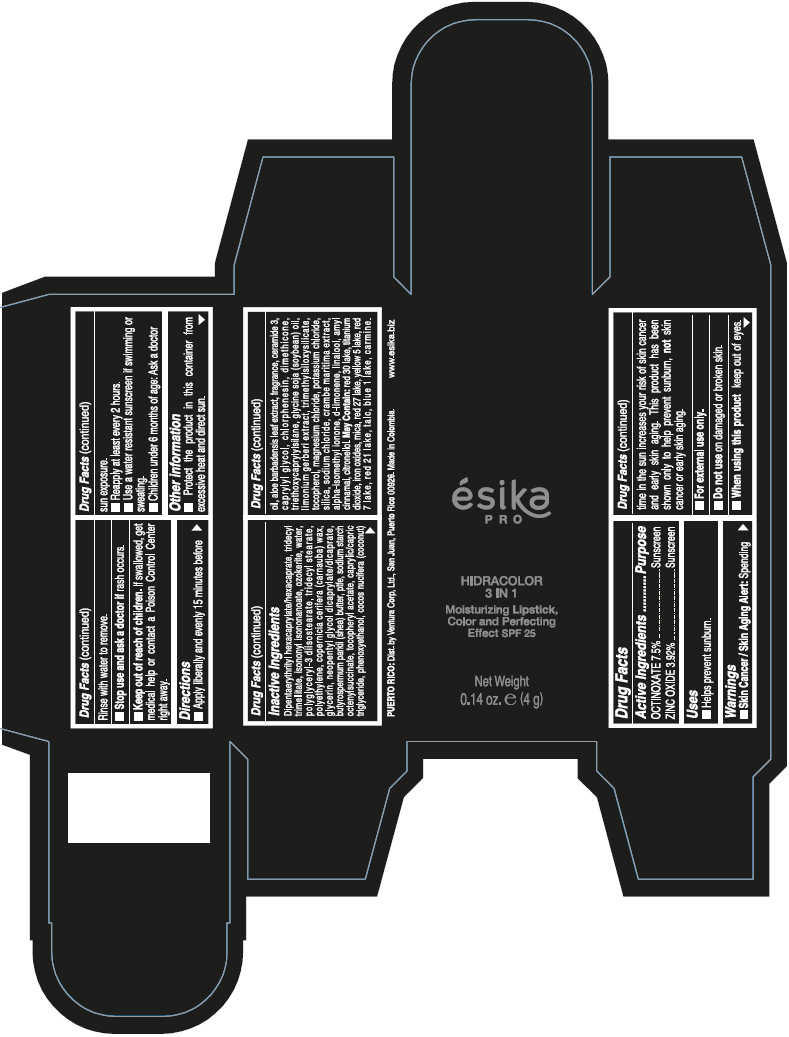
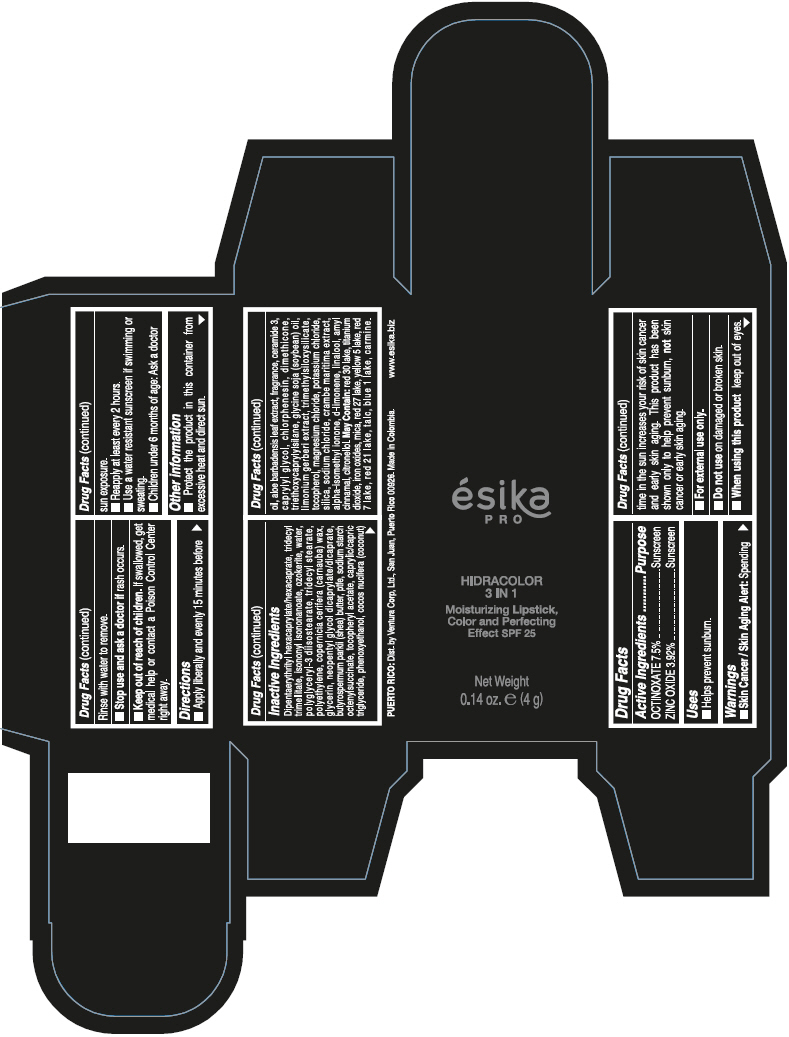
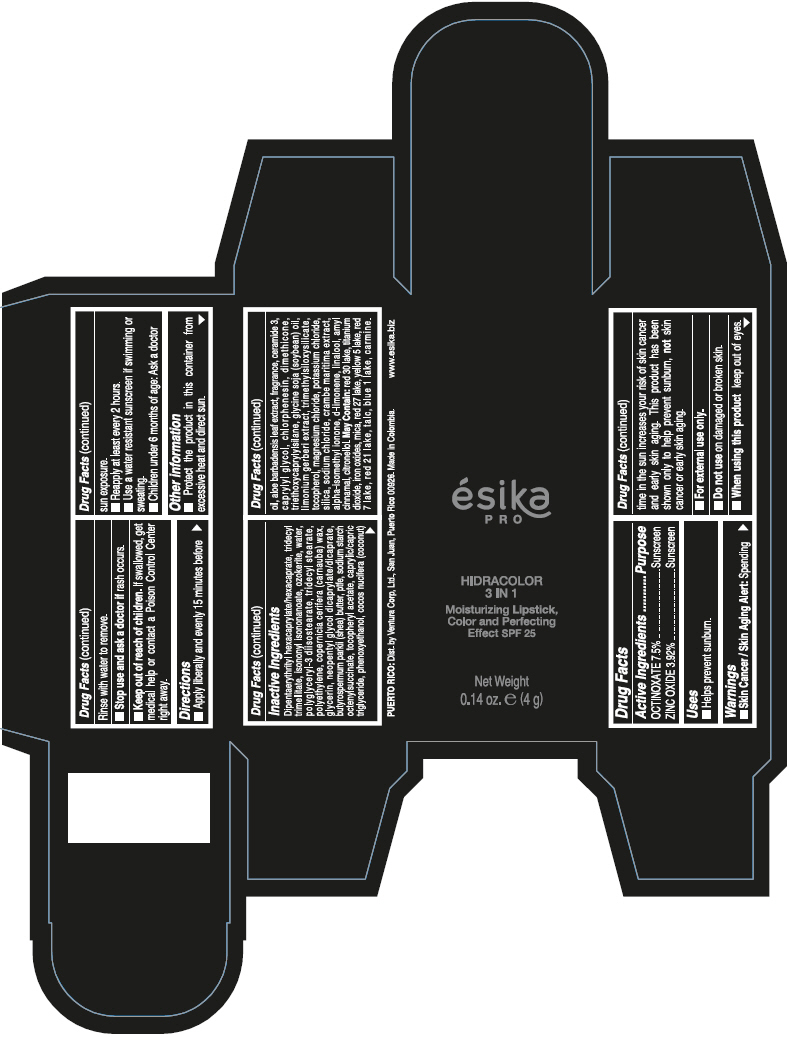
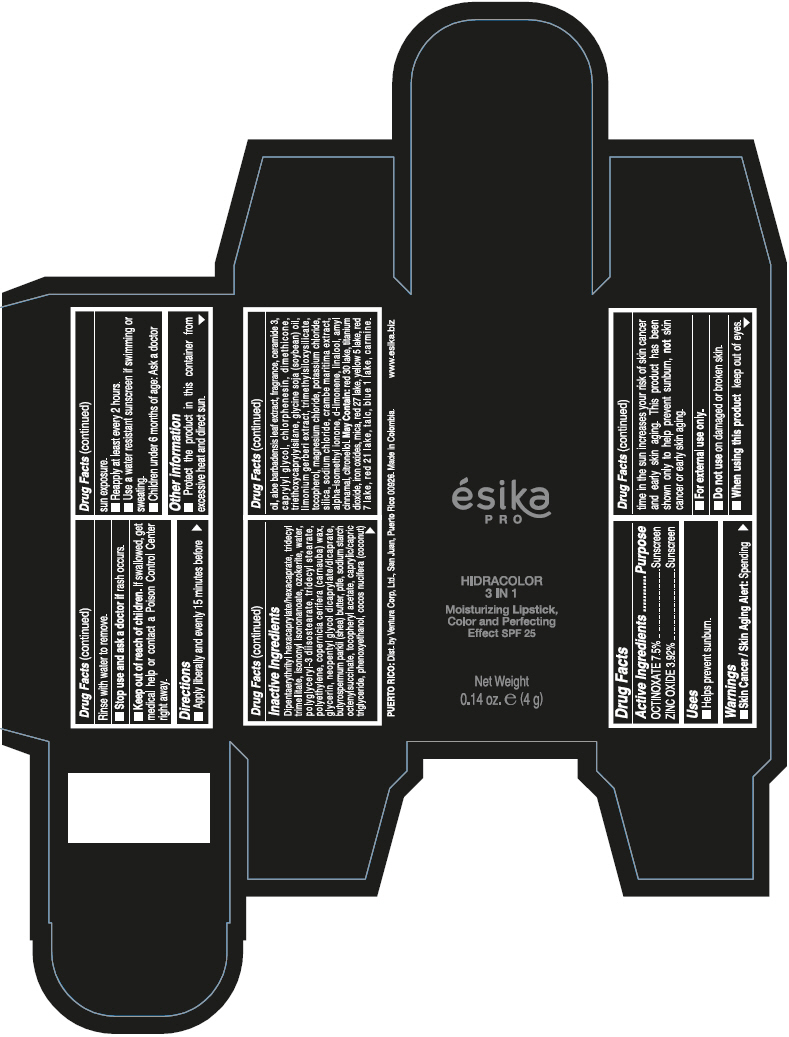
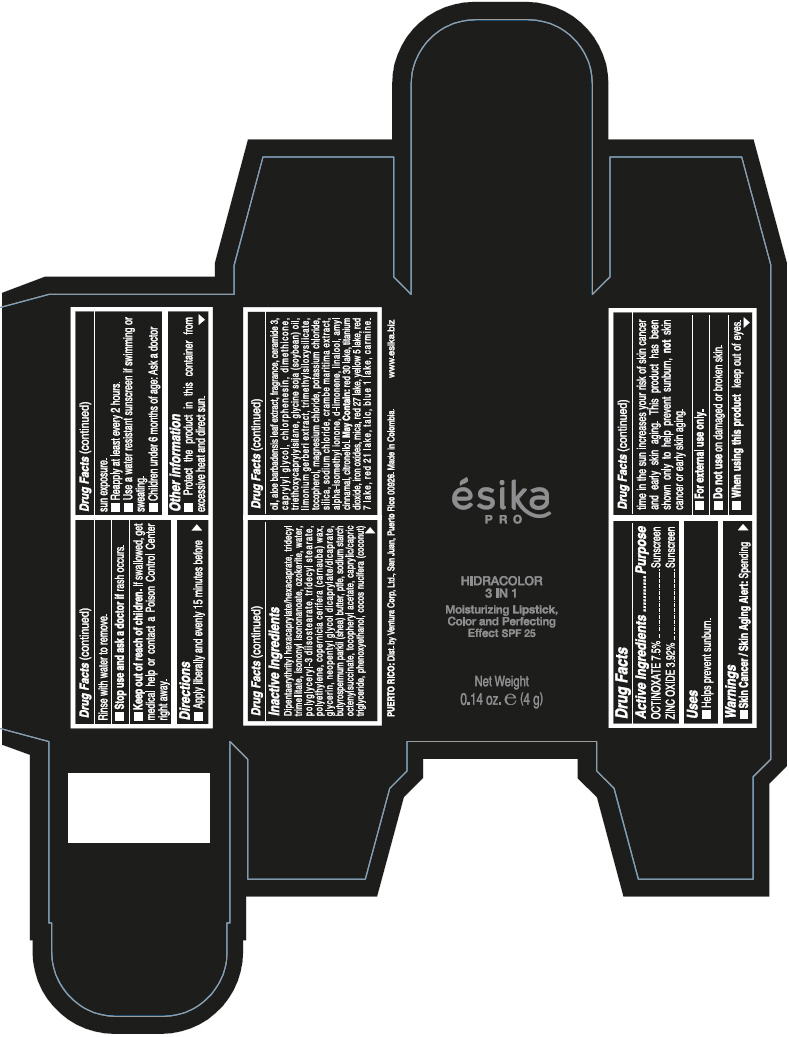
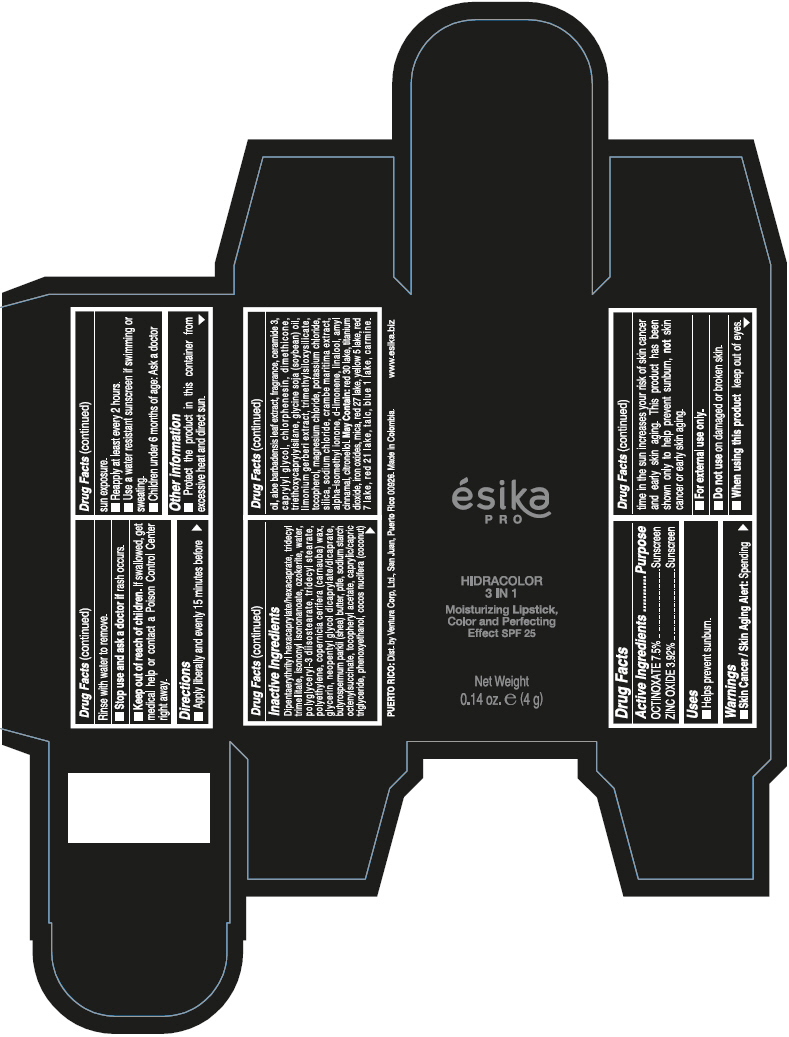
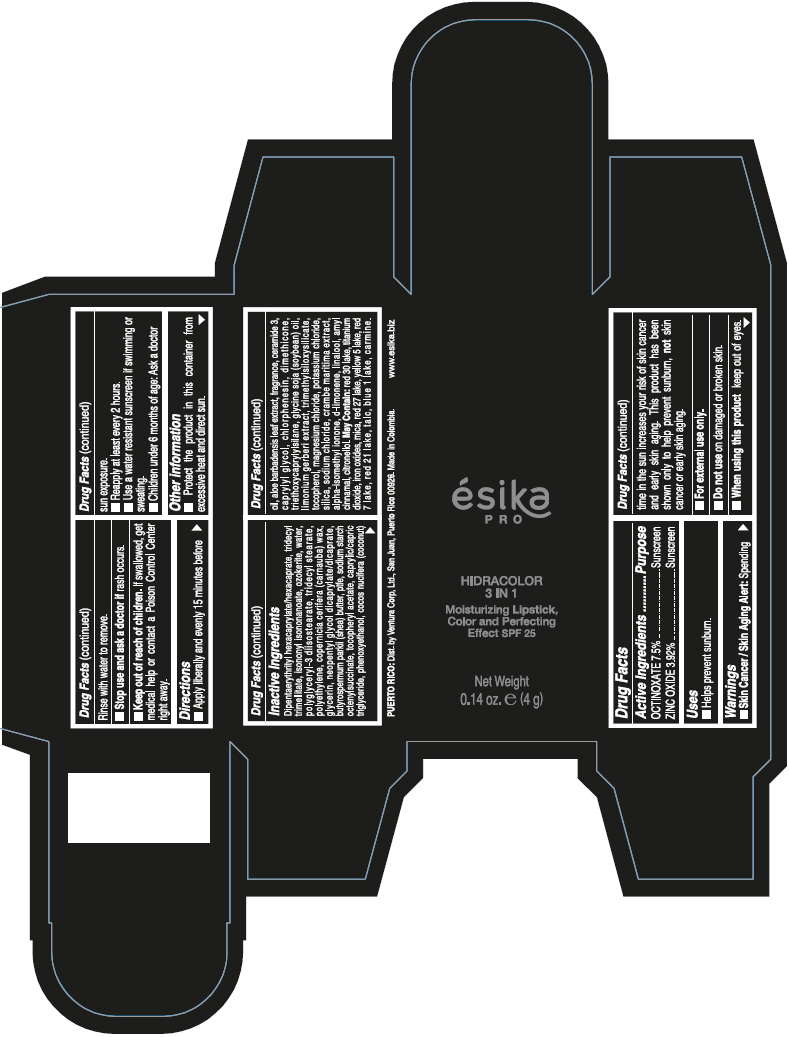
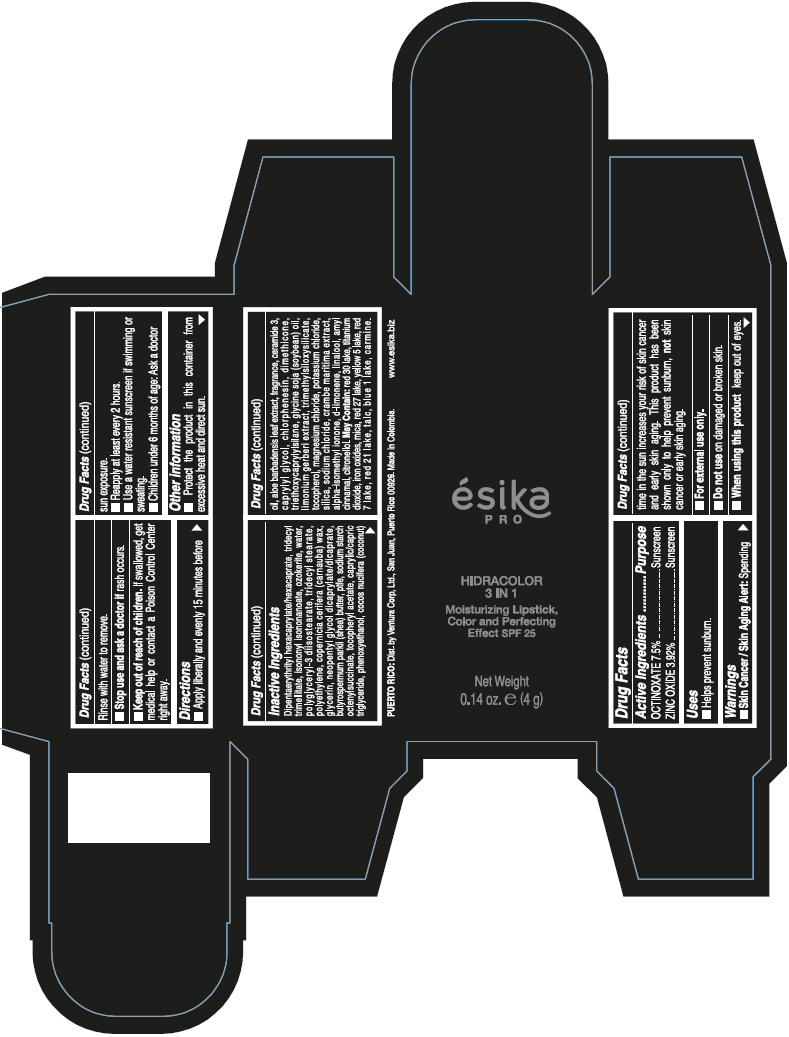
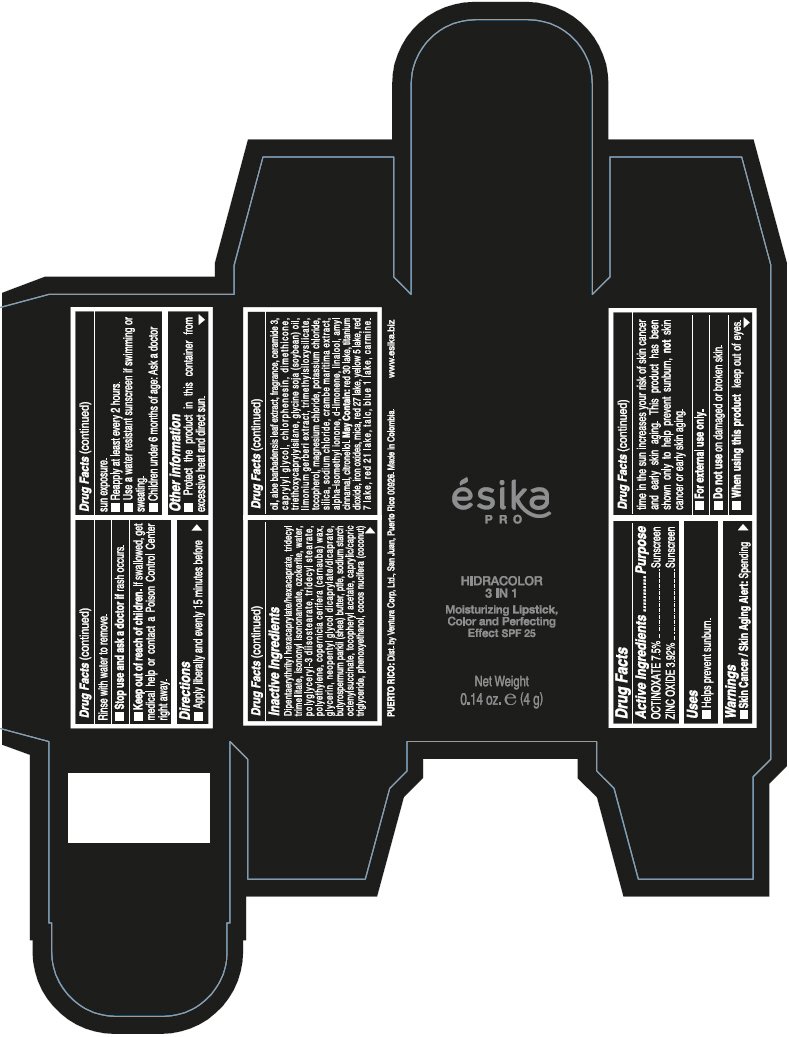
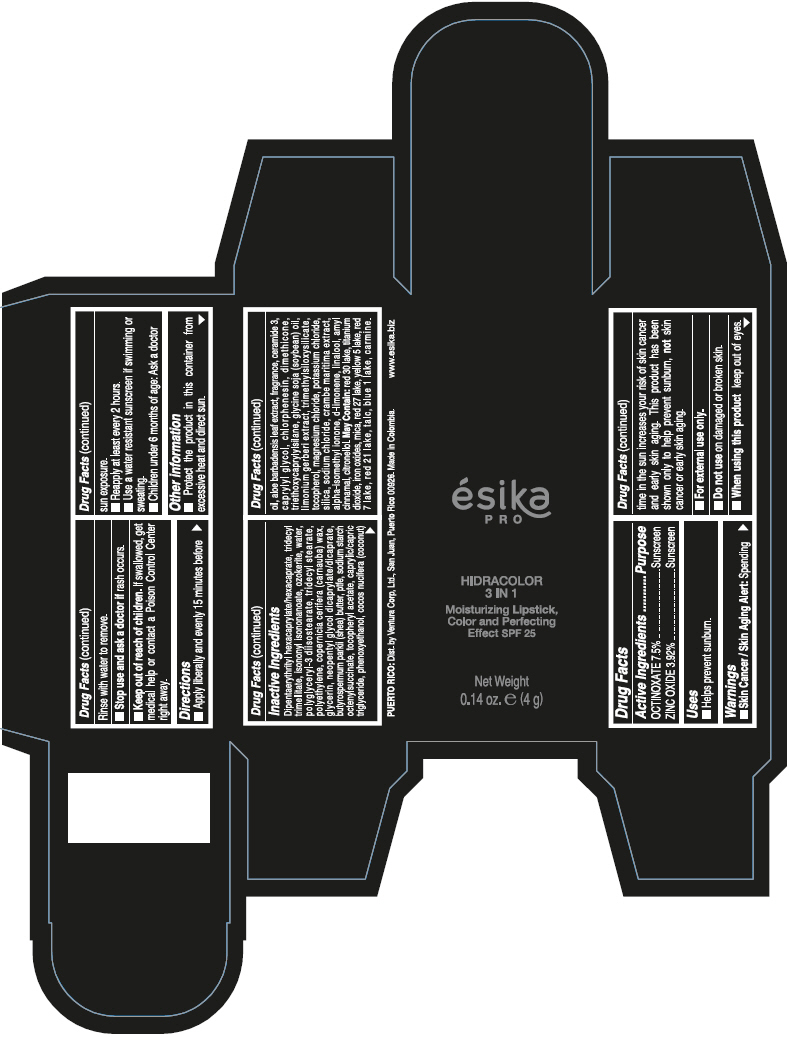
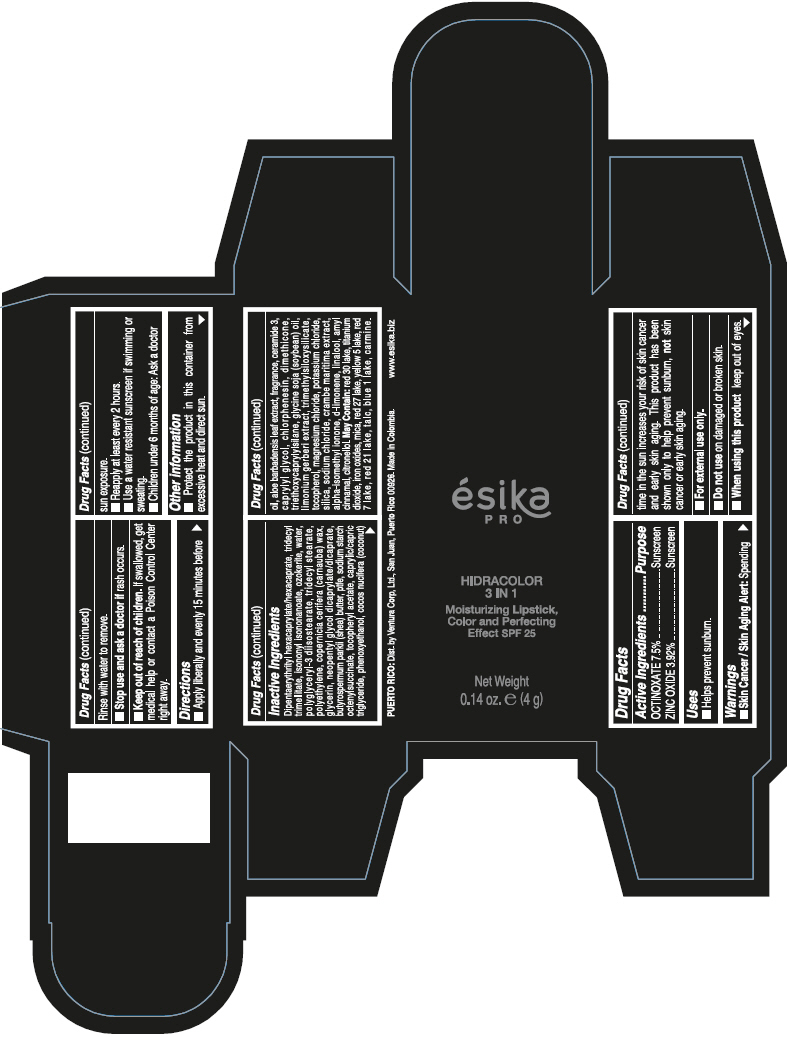
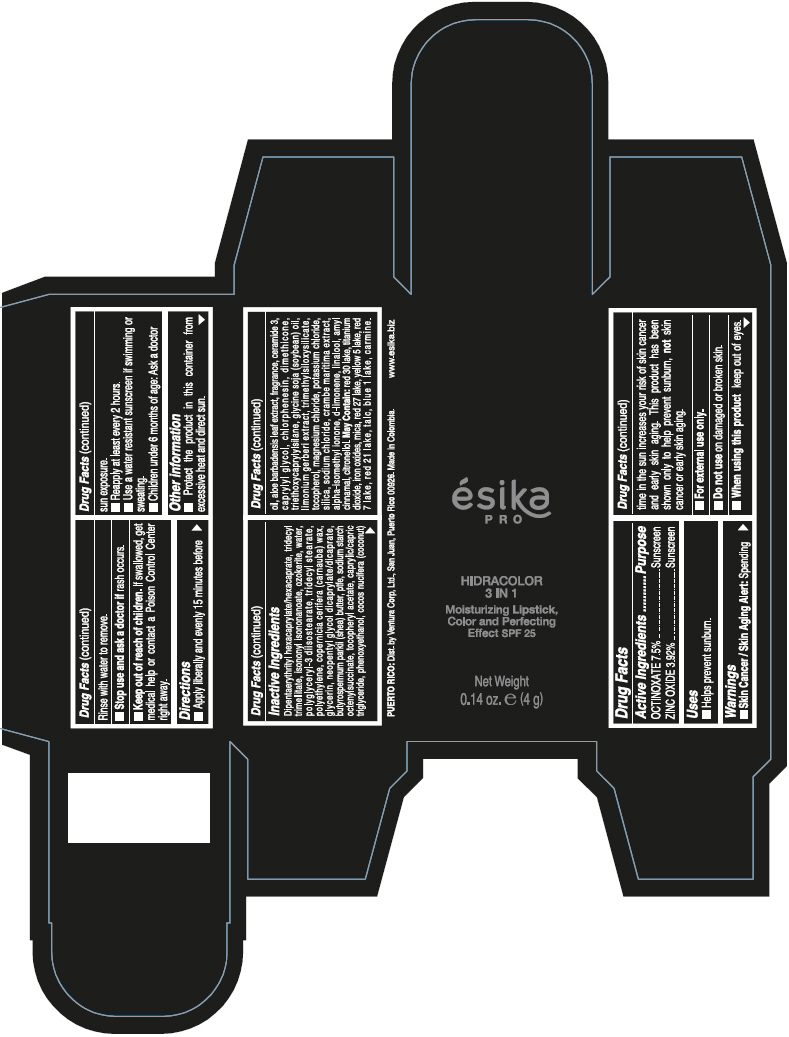
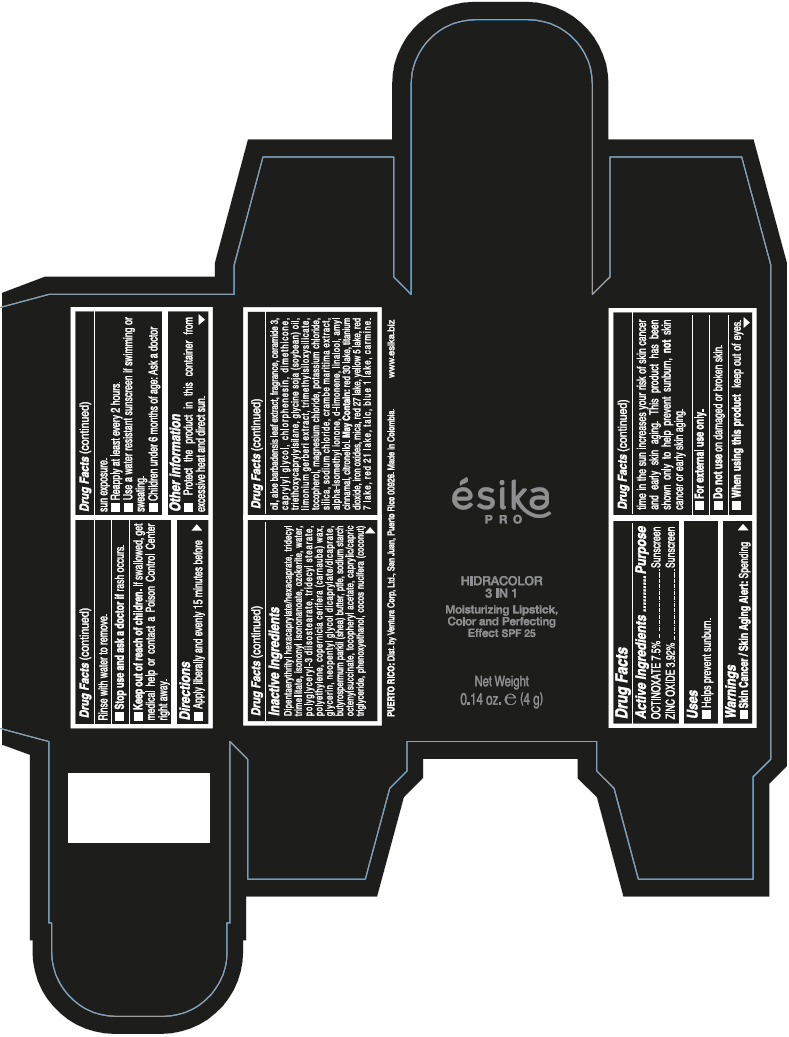
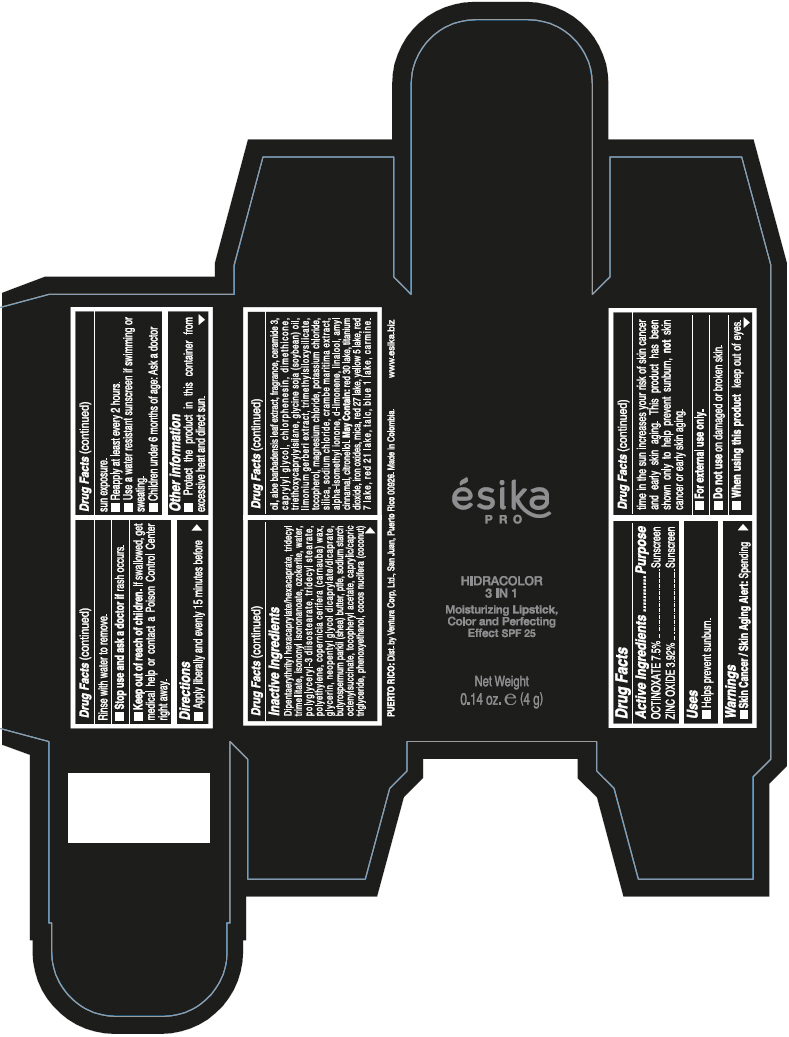
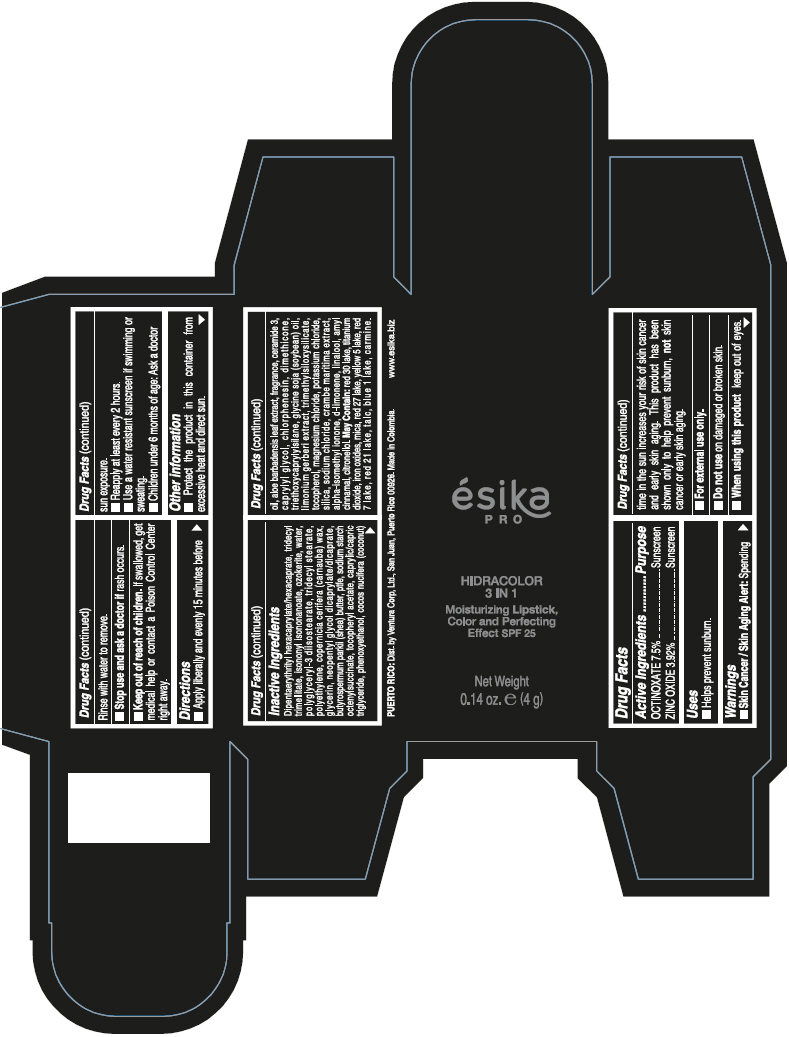
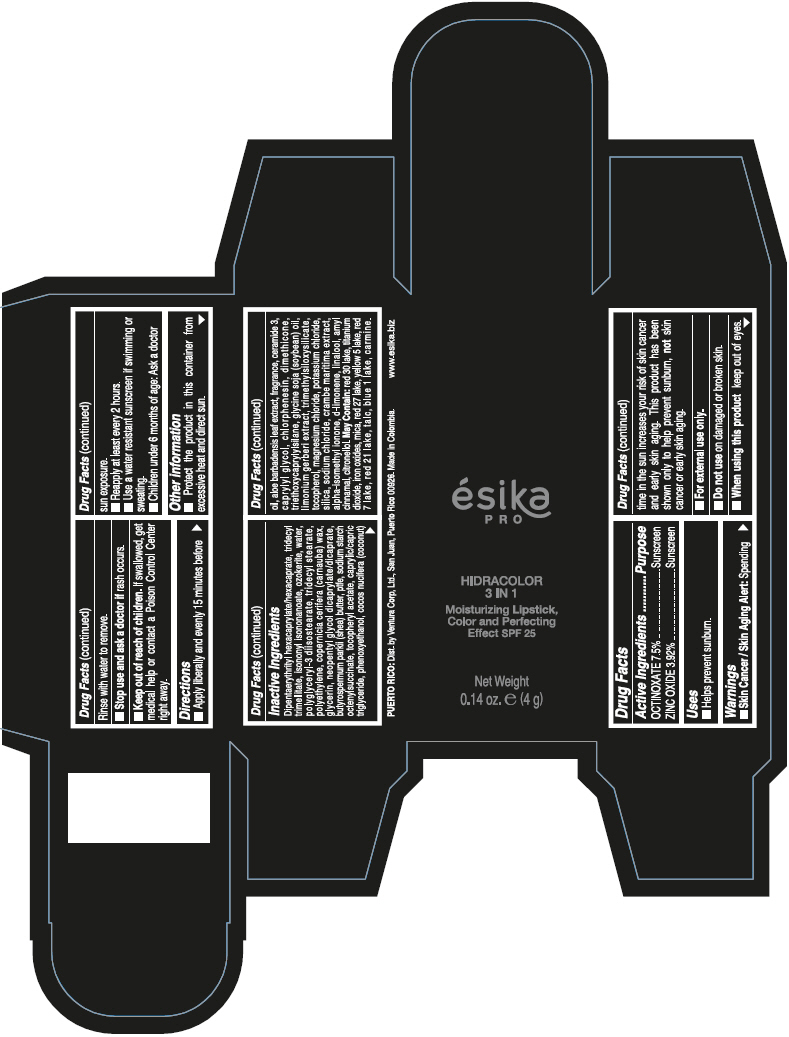
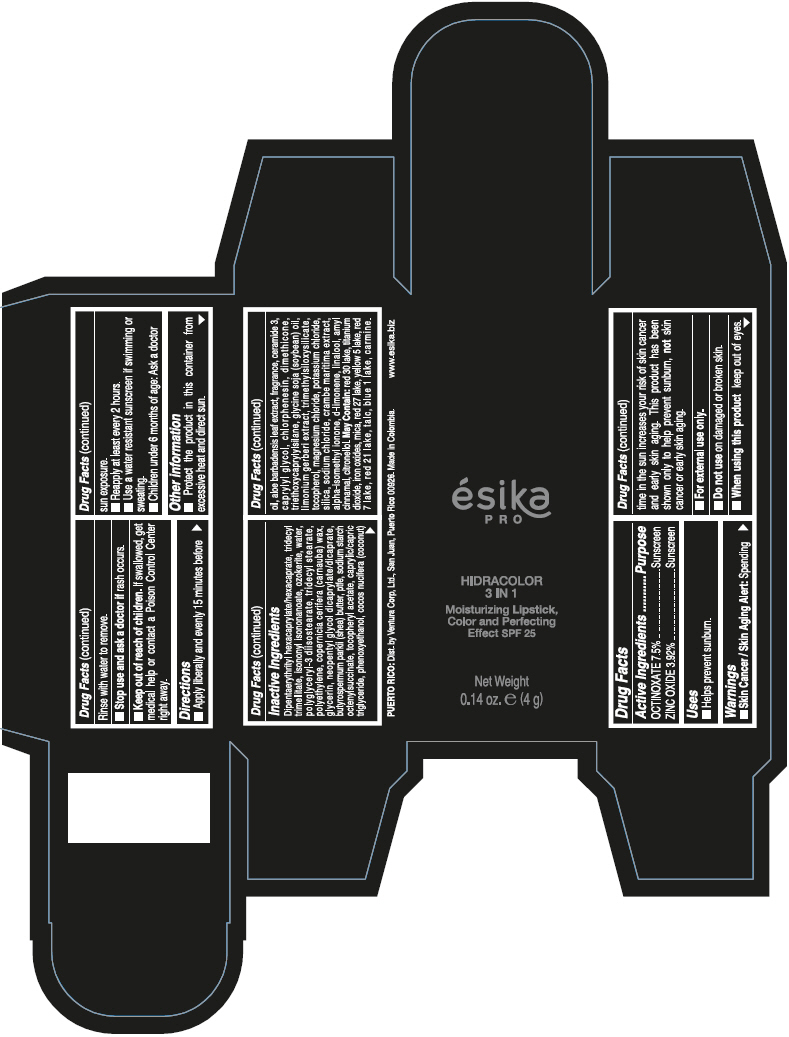
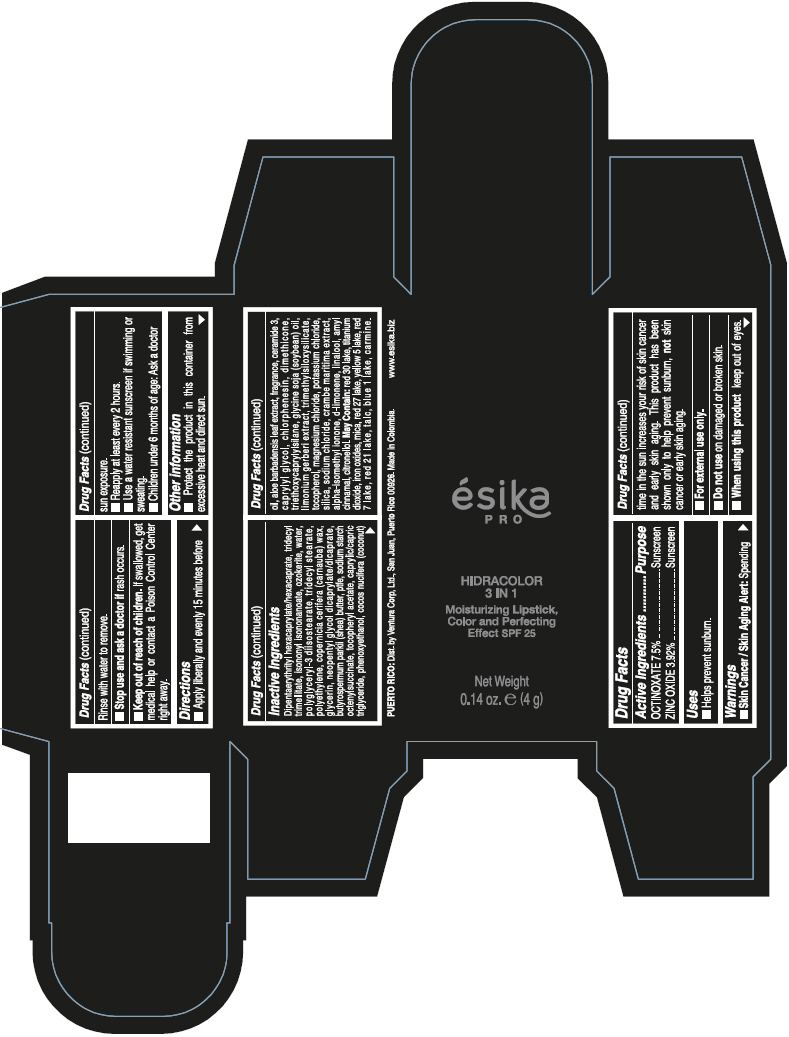
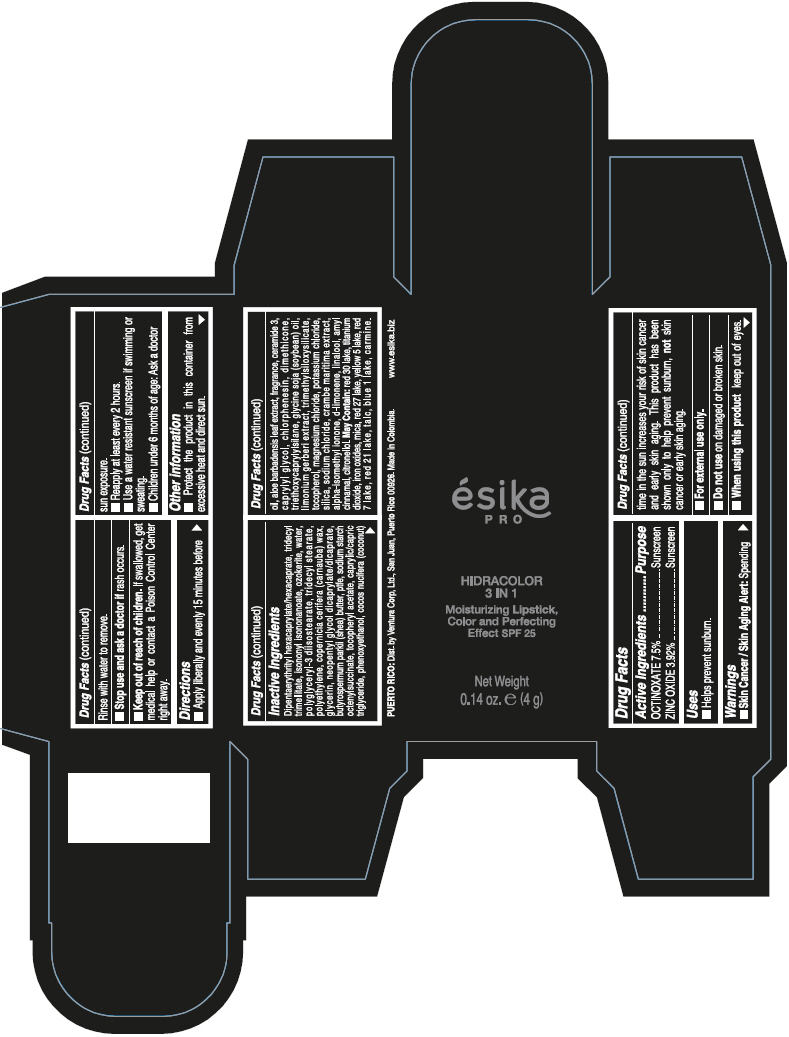
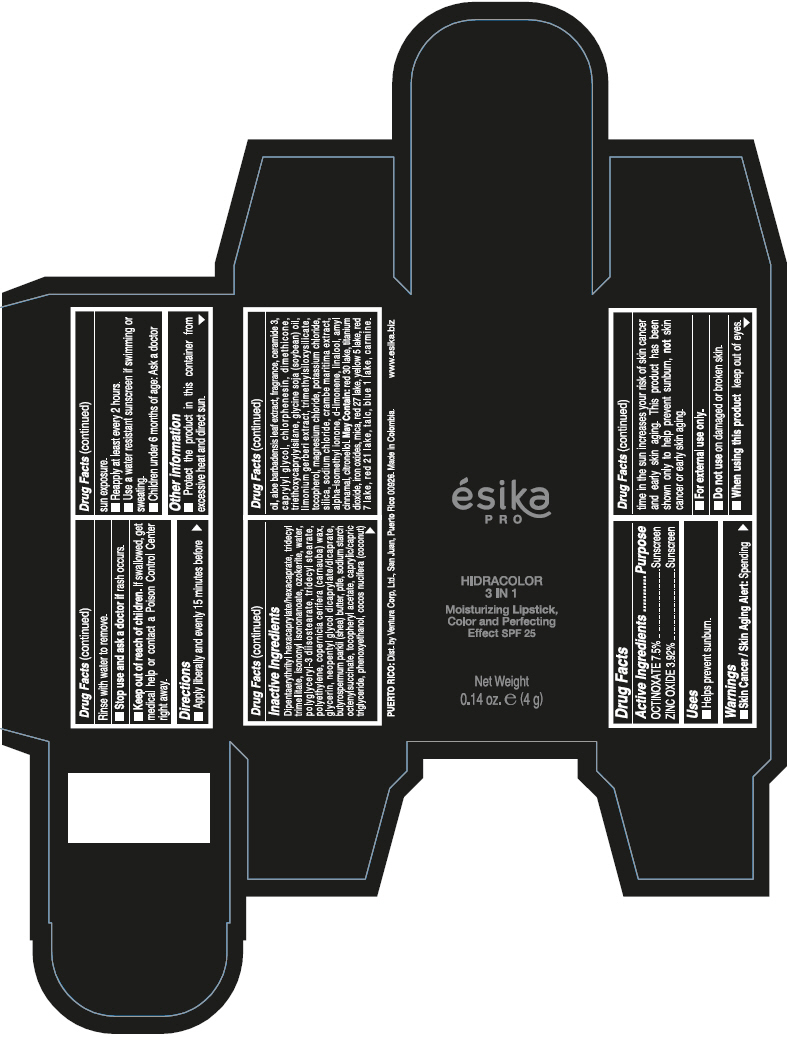
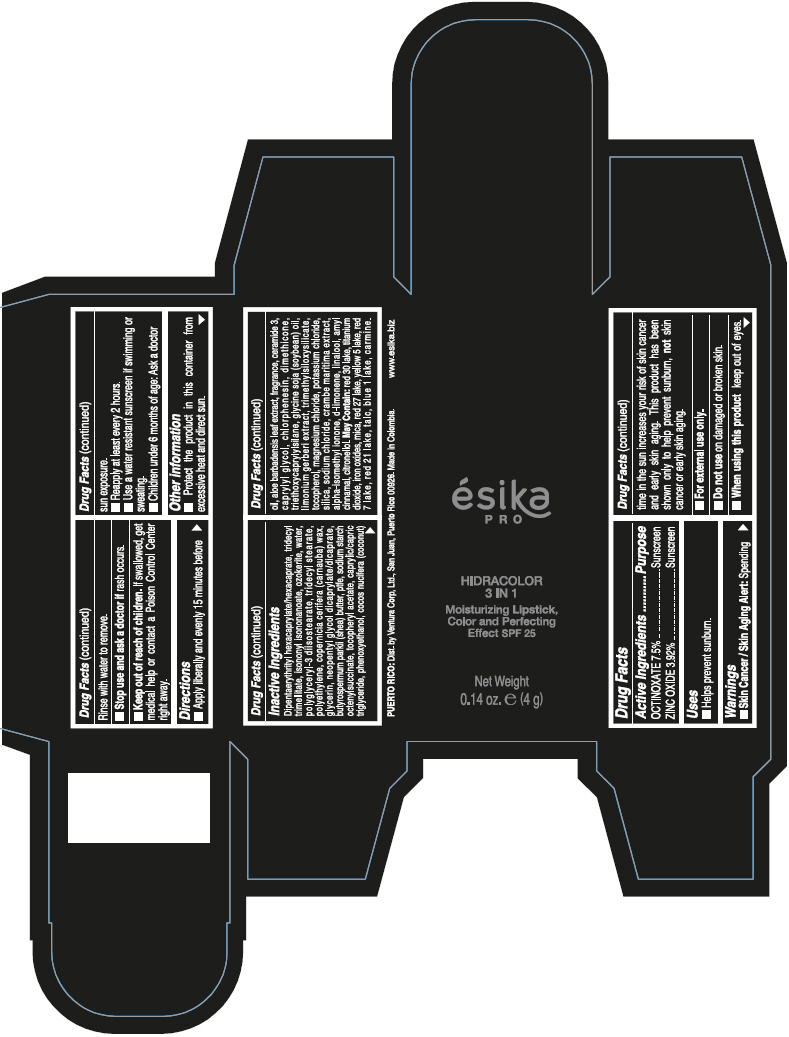
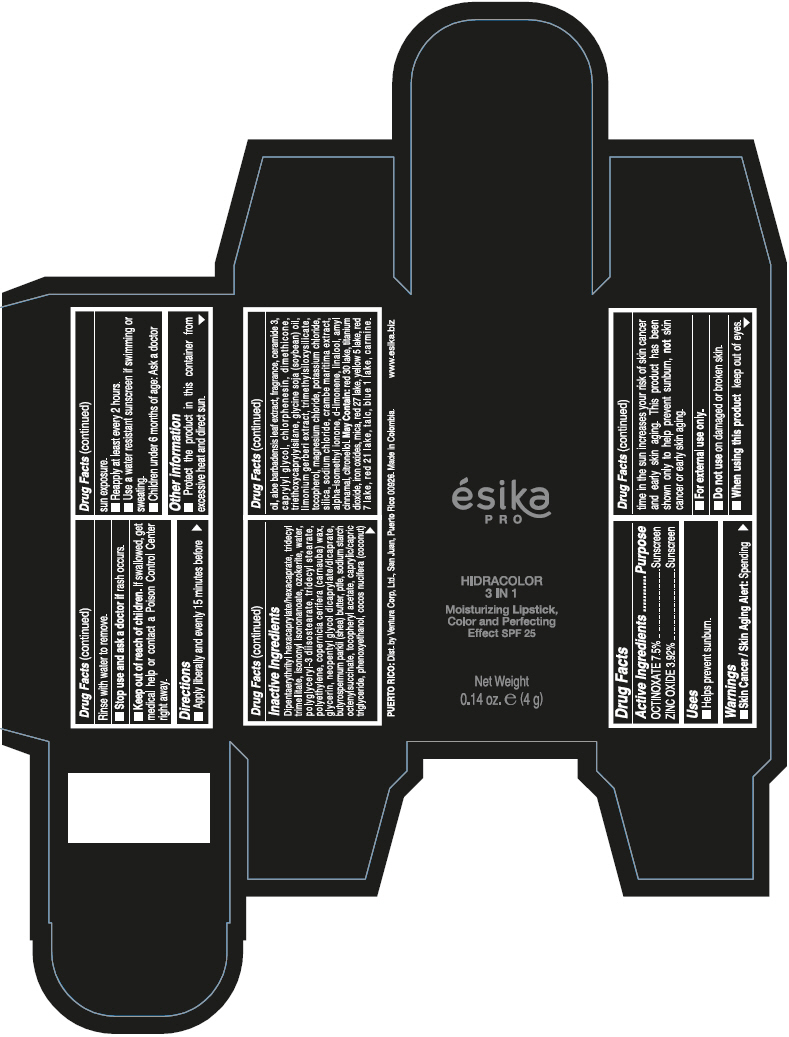
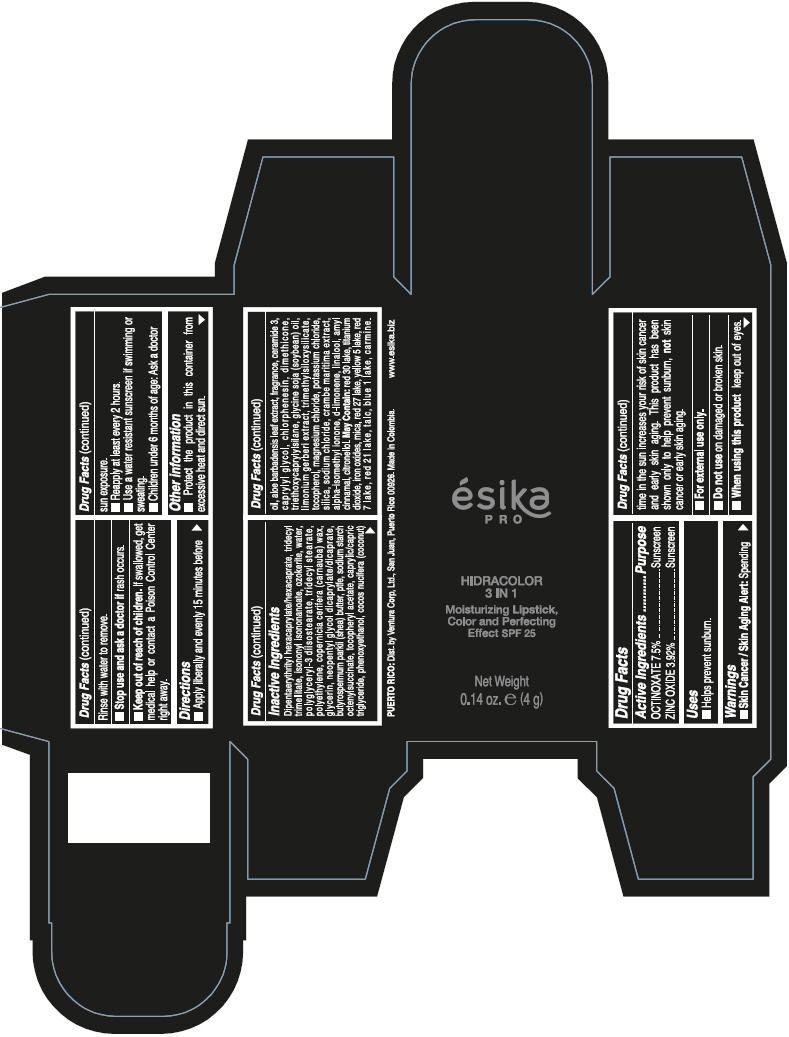
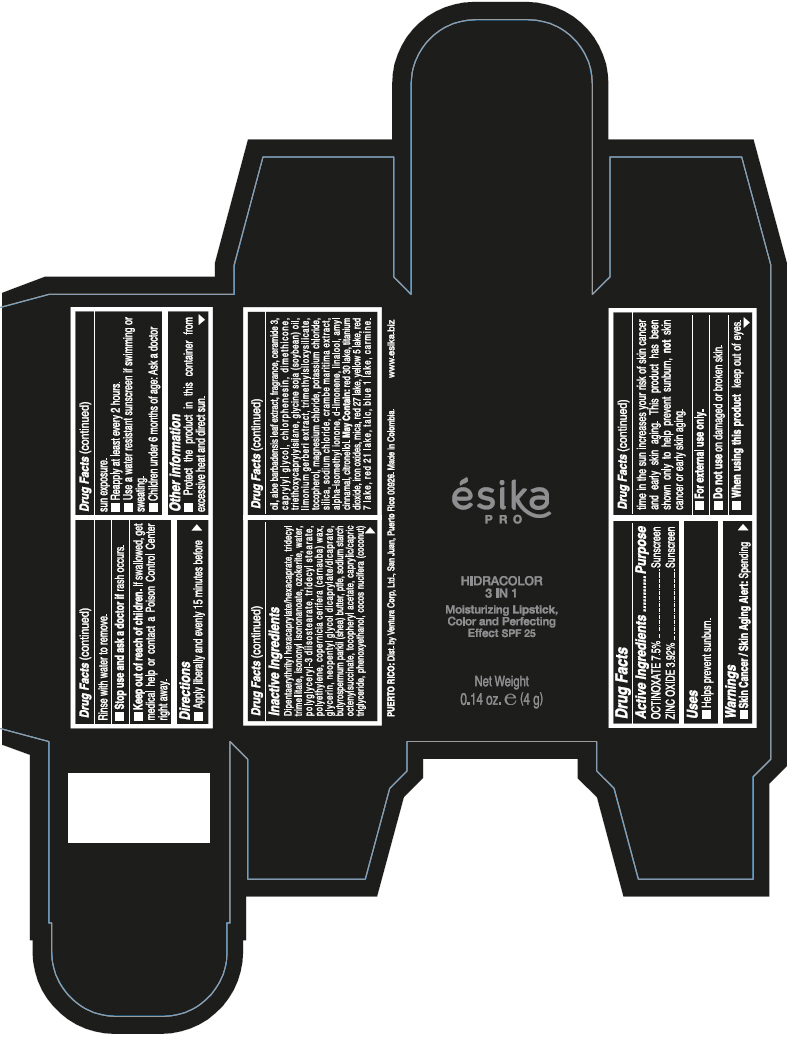
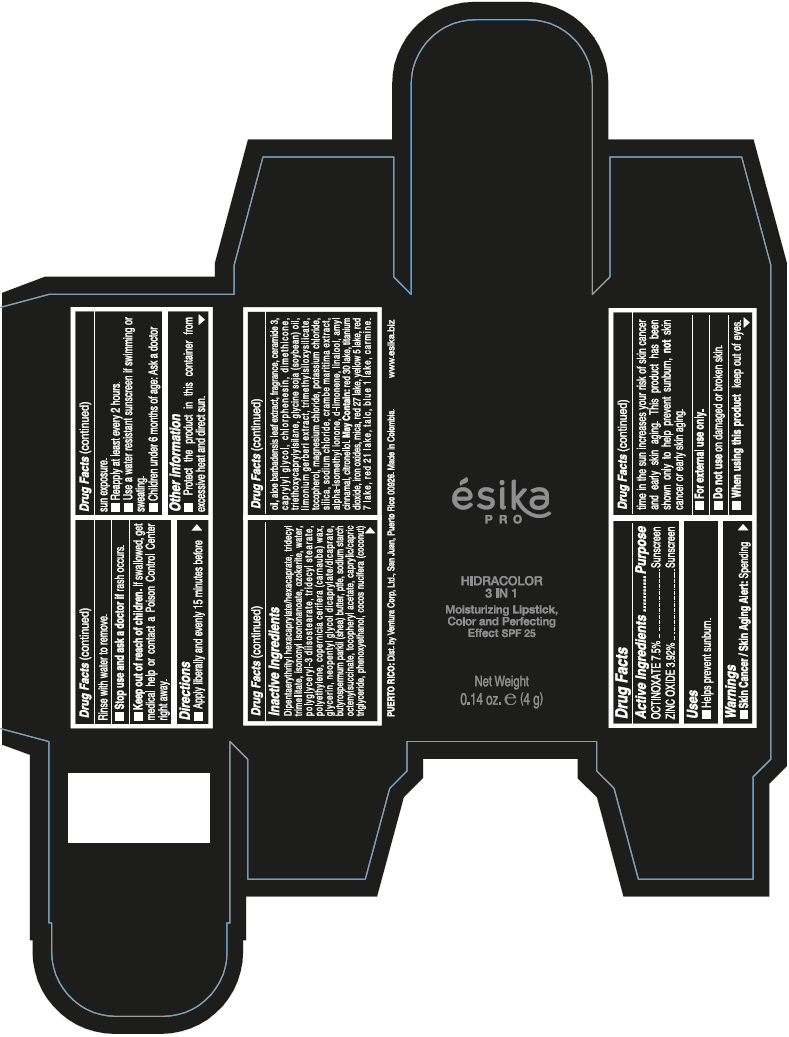
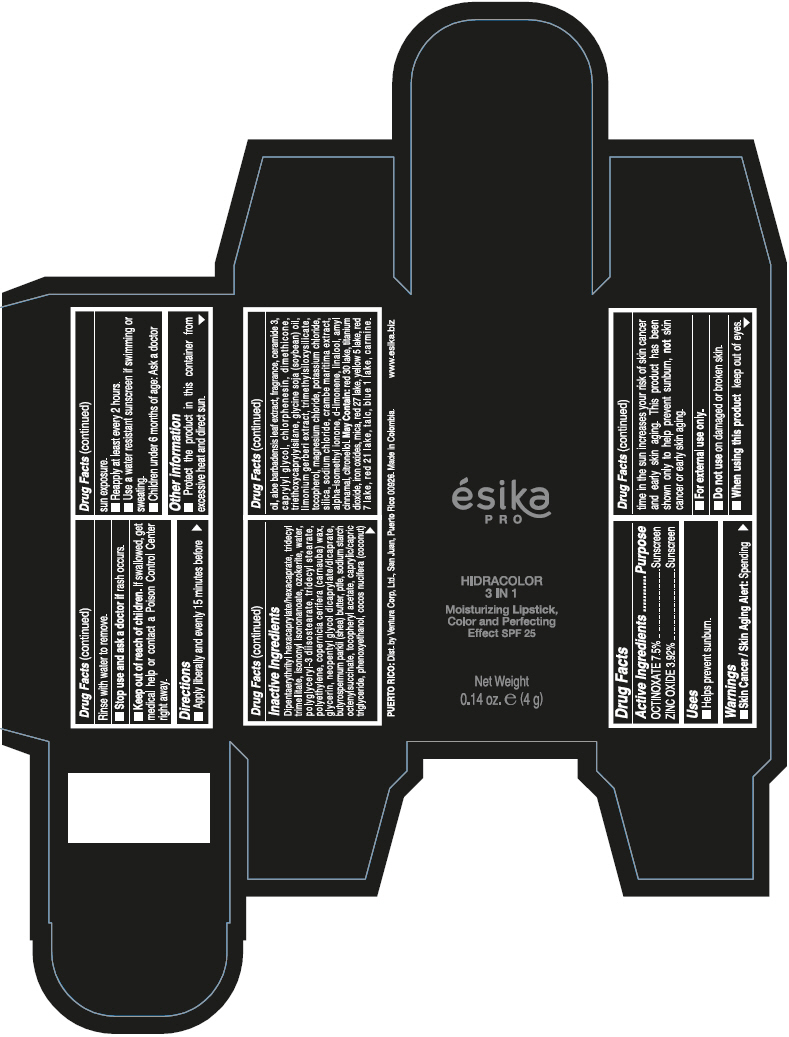
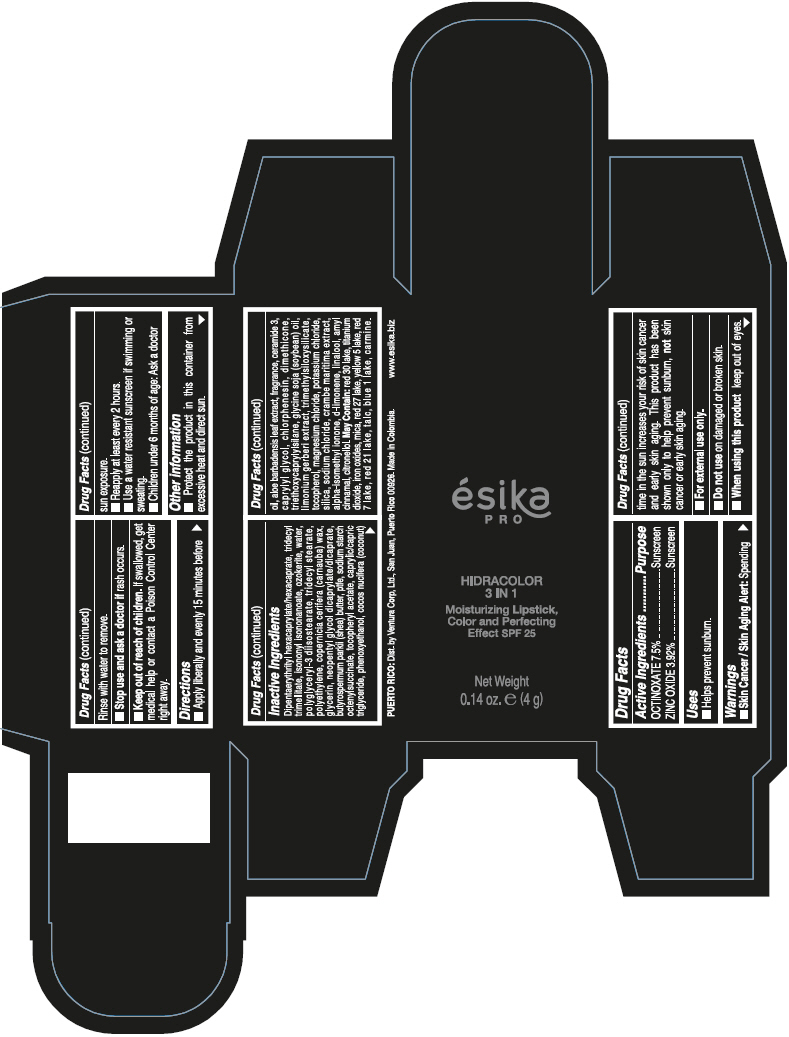
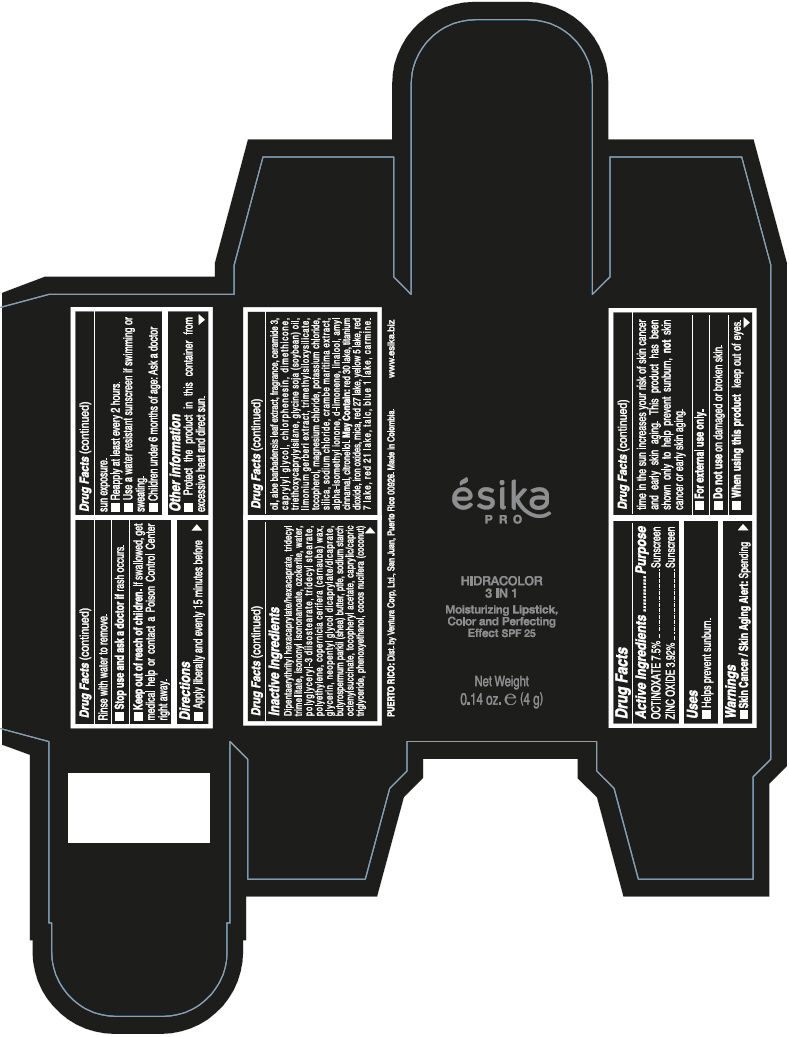
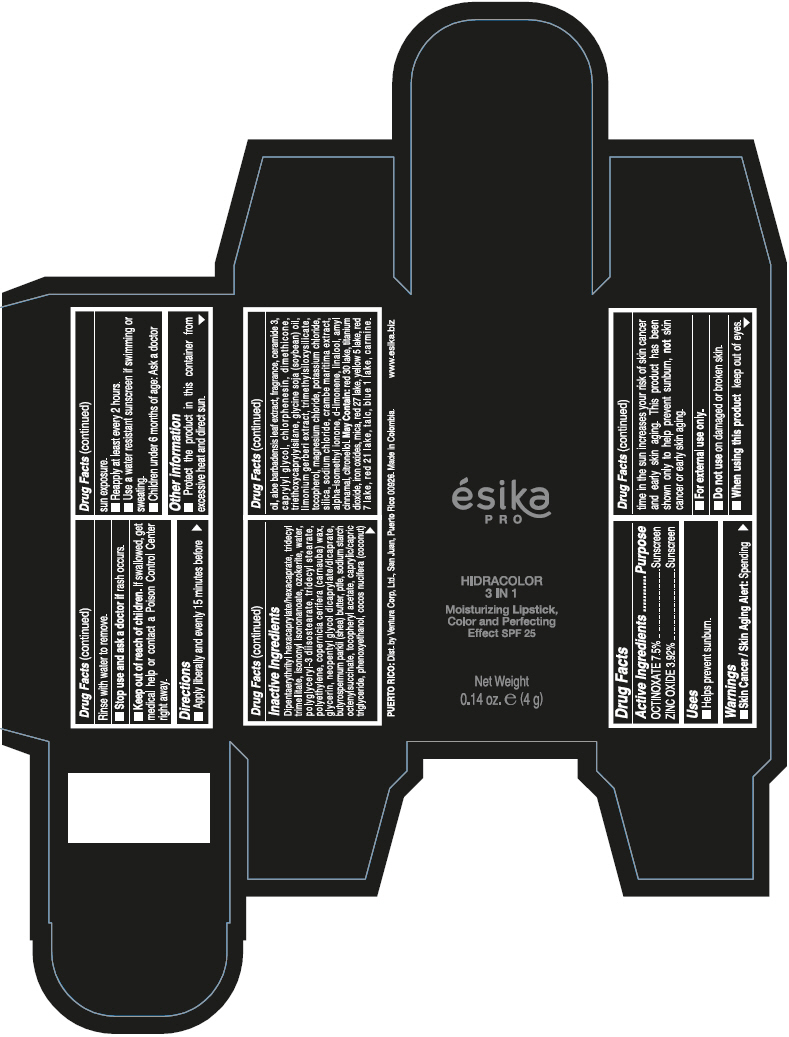
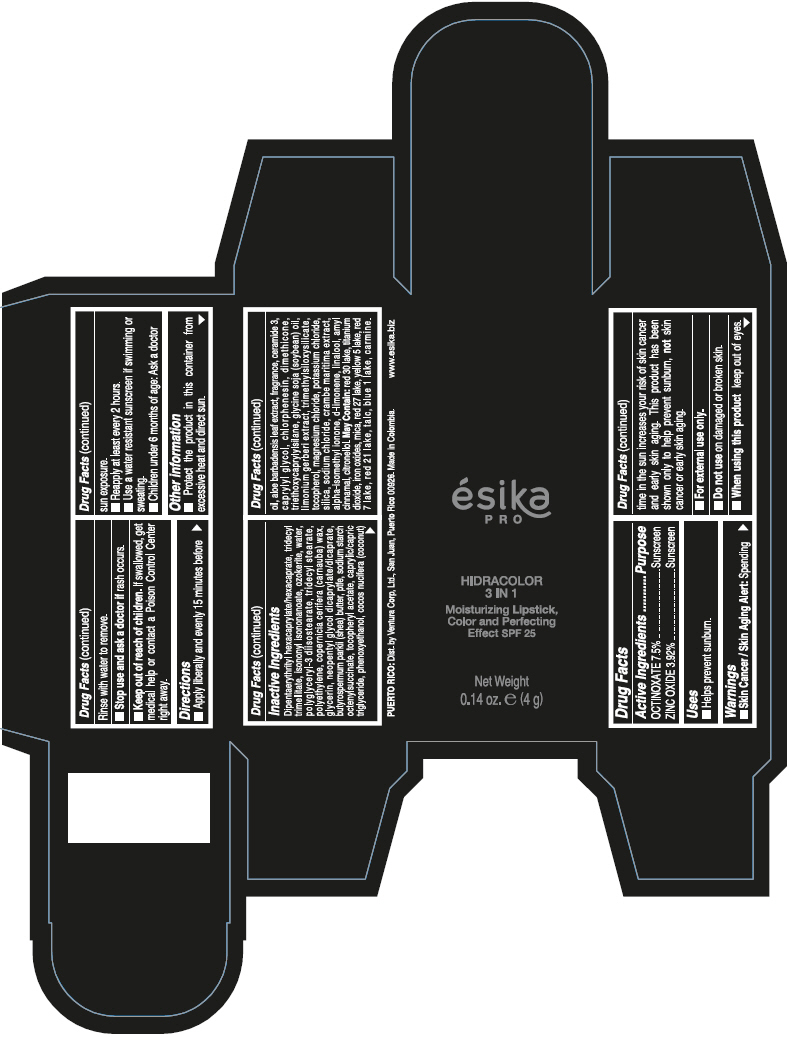
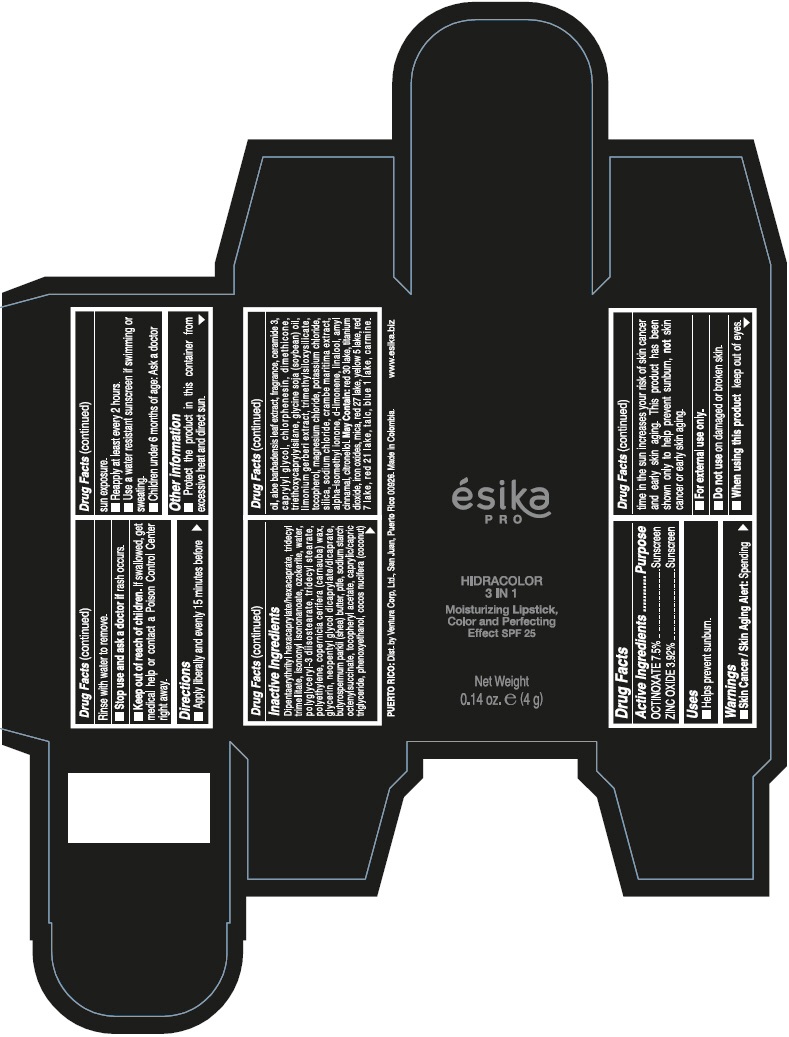
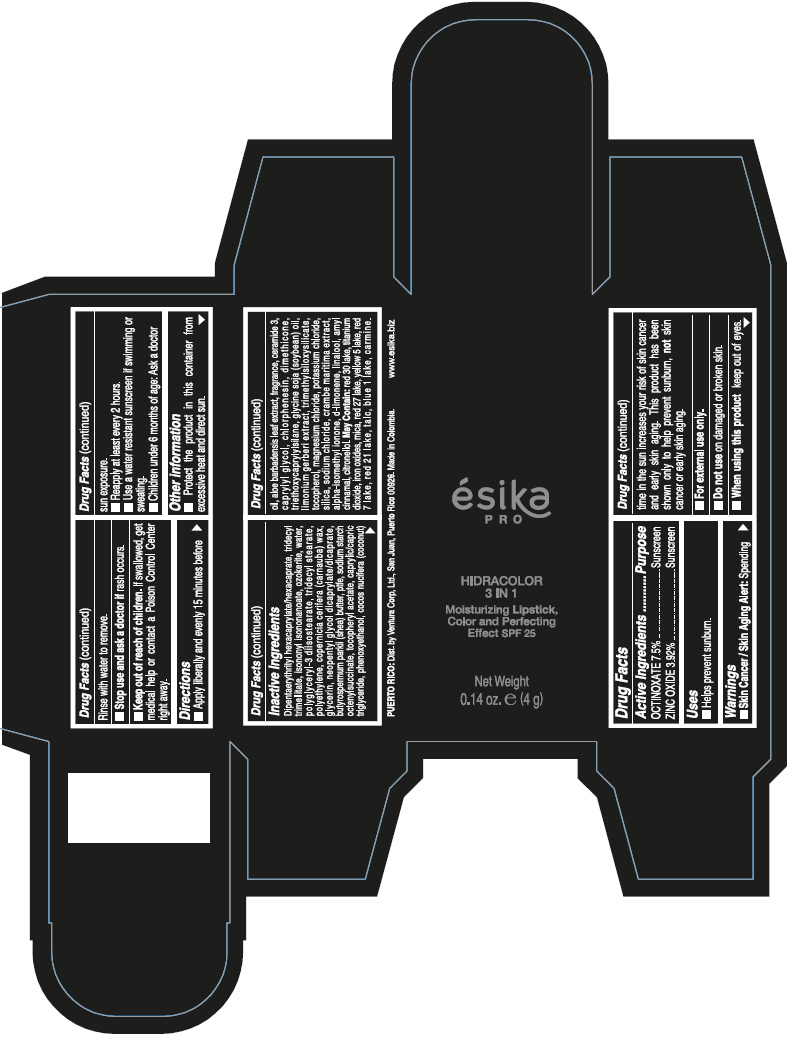
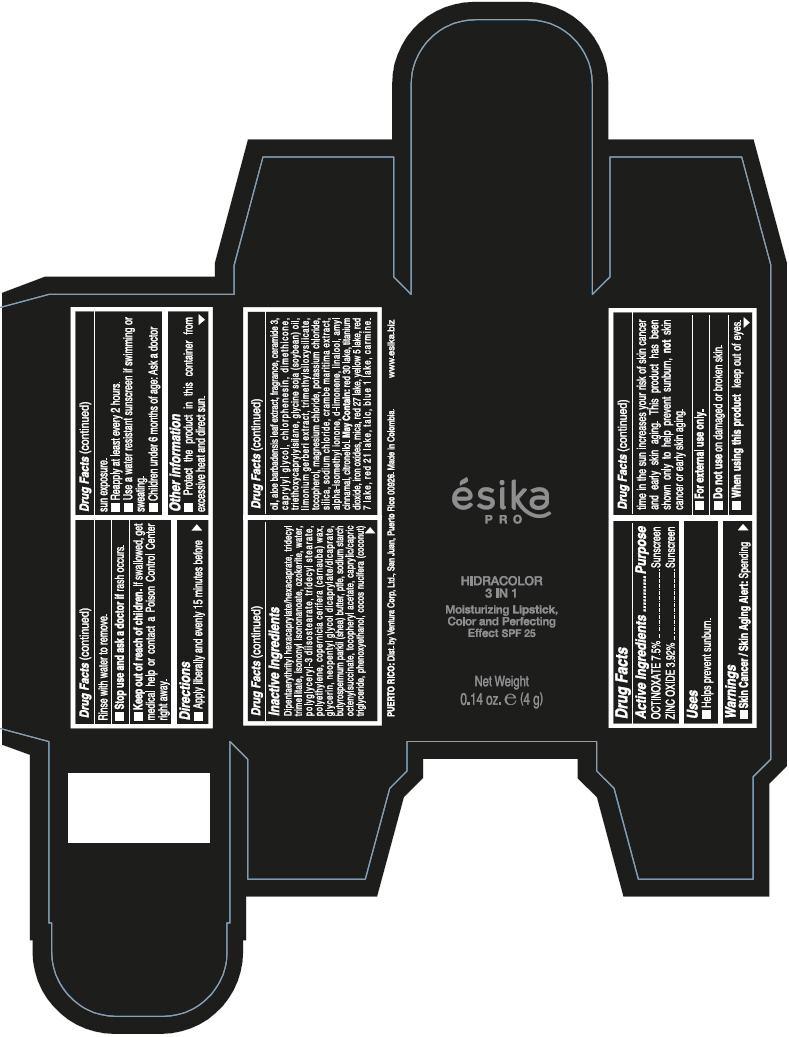
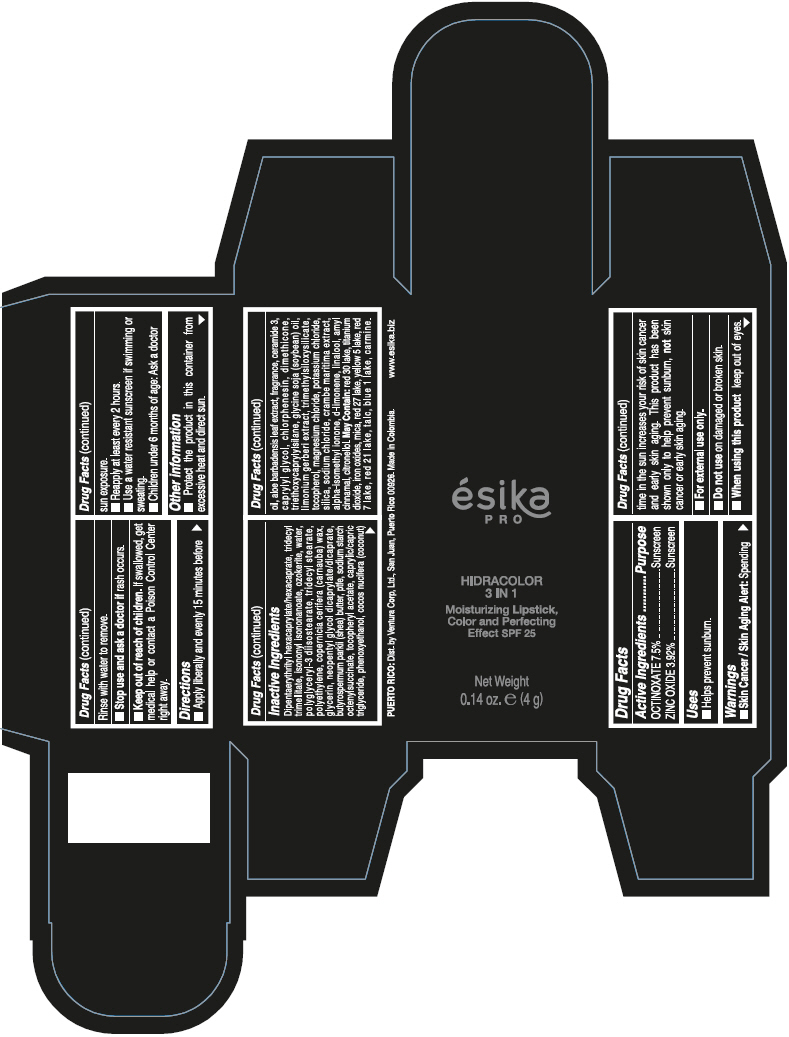
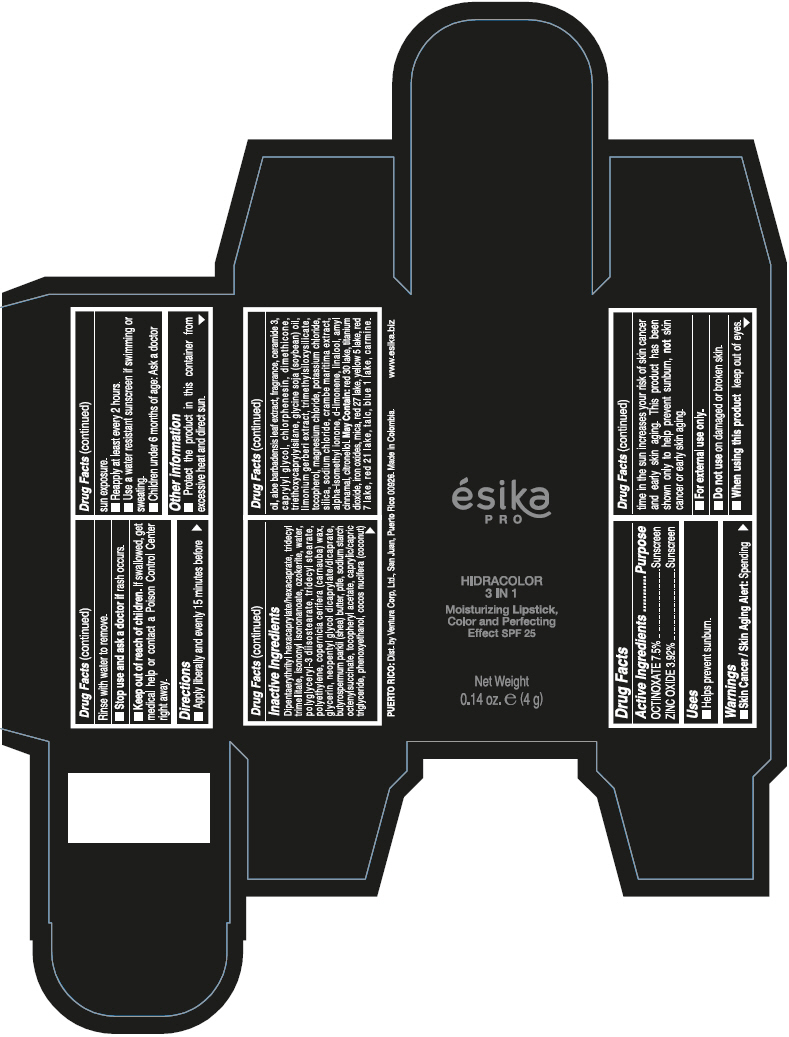
DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE, TRIDECYL TRIMELLITATE, ISONONYL ISONONANOATE, OZOKERITE, WATER, POLYGLYCERYL-3 DIISOSTEARATE, TRIDECYL STEARATE, POLYETHYLENE, COPERNICIA CERIFERA (CARNAUBA) WAX, GLYCERIN, NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE, BUTYROSPERMUM PARKII (SHEA) BUTTER, PTFE, SODIUM STARCH OCTENYLSUCCINATE, TOCOPHERYL ACETATE, CAPRYLIC/CAPRIC TRIGLYCERIDE, PHENOXYETHANOL, COCOS NUCIFERA (COCONUT) OIL, ALOE BARBADENSIS LEAF EXTRACT, FRAGRANCE, CERAMIDE 3, CAPRYLYL GLYCOL, CHLORPHENESIN, DIMETHICONE, TRIETHOXYCAPRYLYLSILANE, GLYCINE SOJA (SOYBEAN) OIL, LIMONIUM GERBERI EXTRACT, TRIMETHYLSILOXYSILICATE, TOCOPHEROL, MAGNESIUM CHLORIDE, POTASSIUM CHLORIDE, SILICA, SODIUM CHLORIDE, CRAMBE MARITIMA EXTRACT, ALPHA-ISOMETHYL IONONE, d-LIMONENE, LINALOOL, AMYL CINNAMAL, CITRONELLOL.
MAY CONTAIN: RED 30 LAKE, TITANIUM DIOXIDE, IRON OXIDES, MICA, RED 27 LAKE, YELLOW 5 LAKE, RED 7 LAKE, RED 21 LAKE, TALC, BLUE 1 LAKE, CARMINE.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (PIMIENTA CALIENTE) - BROWN
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROJO DESEO) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROJO ESIKA) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (MARRON TOPACIO) - BROWN
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROSA GEOMETRIC) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROSA ENCANTO) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (MORA GLAM) - PURPLE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (GRANATE SEDUCTOR) - PURPLE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (BORGONA SEXY) - PURPLE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (FRESA HOT) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (FUCSIA CAUTIVANTE) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (FUCSIA INTENSE) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (NARANJA ESFERA) - ORANGE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROSA NUDE) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (PEACH NUDE) - BROWN
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (CORAL CHIC) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROJO TROPICAL) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (NARANJA CITRICO) - ORANGE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (NUDE PUNCH) - ORANGE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (MORA ATREVIDA) - PURPLE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (NUDE SAVANNA) - ORANGE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROJO SENSUAL) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (NUDE MOCCHA) - ORANGE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (BRONCE METALLIC) - BROWN
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (PIMIENTA CALIENTE) - BROWN
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-958 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-958-02 1 in 1 BOX 07/12/2016 1 NDC:13537-958-01 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROJO DESEO) - RED
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-959 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-959-04 1 in 1 BOX 07/12/2016 1 NDC:13537-959-03 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROJO ESIKA) - RED
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-960 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-960-06 1 in 1 BOX 07/12/2016 1 NDC:13537-960-05 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (MARRON TOPACIO) - BROWN
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-966 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-966-08 1 in 1 BOX 07/12/2016 1 NDC:13537-966-07 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROSA GEOMETRIC) - PINK
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-967 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-967-10 1 in 1 BOX 07/12/2016 1 NDC:13537-967-09 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROSA ENCANTO) - PINK
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-968 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-968-12 1 in 1 BOX 07/12/2016 1 NDC:13537-968-11 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (MORA GLAM) - PURPLE
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-969 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-969-14 1 in 1 BOX 07/12/2016 1 NDC:13537-969-13 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (GRANATE SEDUCTOR) - PURPLE
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-970 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-970-18 1 in 1 BOX 07/12/2016 1 NDC:13537-970-17 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (BORGONA SEXY) - PURPLE
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-971 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-971-20 1 in 1 BOX 07/12/2016 1 NDC:13537-971-19 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (FRESA HOT) - PINK
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-972 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-972-22 1 in 1 BOX 07/12/2016 1 NDC:13537-972-21 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (FUCSIA CAUTIVANTE) - PINK
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-973 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-973-24 1 in 1 BOX 07/12/2016 1 NDC:13537-973-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (FUCSIA INTENSE) - PINK
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-974 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-974-24 1 in 1 BOX 07/12/2016 1 NDC:13537-974-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (NARANJA ESFERA) - ORANGE
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-975 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-975-24 1 in 1 BOX 07/12/2016 1 NDC:13537-975-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROSA NUDE) - PINK
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-976 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-976-24 1 in 1 BOX 07/12/2016 1 NDC:13537-976-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (PEACH NUDE) - BROWN
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-977 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-977-24 1 in 1 BOX 07/12/2016 1 NDC:13537-977-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (CORAL CHIC) - PINK
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-978 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-978-24 1 in 1 BOX 07/12/2016 1 NDC:13537-978-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROJO TROPICAL) - RED
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-979 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-979-24 1 in 1 BOX 07/12/2016 1 NDC:13537-979-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (NARANJA CITRICO) - ORANGE
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-980 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-980-24 1 in 1 BOX 07/12/2016 1 NDC:13537-980-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (NUDE PUNCH) - ORANGE
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-981 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-981-24 1 in 1 BOX 07/12/2016 1 NDC:13537-981-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (MORA ATREVIDA) - PURPLE
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-982 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-982-24 1 in 1 BOX 07/12/2016 1 NDC:13537-982-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (NUDE SAVANNA) - ORANGE
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-983 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-983-24 1 in 1 BOX 07/12/2016 1 NDC:13537-983-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROJO SENSUAL) - RED
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-984 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-984-24 1 in 1 BOX 07/12/2016 1 NDC:13537-984-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (NUDE MOCCHA) - ORANGE
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-985 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-985-24 1 in 1 BOX 07/12/2016 1 NDC:13537-985-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (BRONCE METALLIC) - BROWN
octinoxate and zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-986 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-986-24 1 in 1 BOX 07/12/2016 1 NDC:13537-986-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25
octinoxate and zinc oxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-987 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-987-28 3 in 1 BOX 07/12/2016 1 NDC:13537-987-27 1 in 1 TUBE Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 8 g Part 2 1 TUBE 8 g Part 3 1 TUBE 8 g Part 4 1 TUBE 8 g Part 5 1 TUBE 8 g Part 6 1 TUBE 8 g Part 7 1 TUBE 8 g Part 8 1 TUBE 8 g Part 9 1 TUBE 8 g Part 10 1 TUBE 8 g Part 11 1 TUBE 8 g Part 12 1 TUBE 8 g Part 13 1 TUBE 8 g Part 14 1 TUBE 8 g Part 15 1 TUBE 8 g Part 16 1 TUBE 8 g Part 17 1 TUBE 8 g Part 18 1 TUBE 8 g Part 19 1 TUBE 8 g Part 20 1 TUBE 8 g Part 21 1 TUBE 8 g Part 22 1 TUBE 8 g Part 23 1 TUBE 8 g Part 24 1 TUBE 8 g Part 1 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (PIMIENTA CALIENTE) - BROWN
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 2 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROJO DESEO) - RED
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 3 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROJO ESIKA) - RED
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 4 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (MARRON TOPACIO) - BROWN
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 5 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROSA GEOMETRIC) - PINK
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 6 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROSA ENCANTO) - PINK
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 7 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (MORA GLAM) - PURPLE
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 8 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (GRANATE SEDUCTOR) - PURPLE
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 9 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (BORGONA SEXY) - PURPLE
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 10 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (FRESA HOT) - PINK
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 11 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (FUCSIA CAUTIVANTE) - PINK
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 12 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (FUCSIA INTENSE) - PINK
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 13 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (NARANJA ESFERA) - ORANGE
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 14 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROSA NUDE) - PINK
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 15 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (PEACH NUDE) - BROWN
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 16 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (CORAL CHIC) - PINK
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 17 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROJO TROPICAL) - RED
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 18 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (NARANJA CITRICO) - ORANGE
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 19 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (NUDE PUNCH) - ORANGE
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 20 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (MORA ATREVIDA) - PURPLE
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 21 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (NUDE SAVANNA) - ORANGE
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 22 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (ROJO SENSUAL) - RED
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 23 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (NUDE MOCCHA) - ORANGE
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Part 24 of 24 ESIKA PRO HIDRACOLOR 3 IN 1 MOISTURIZING, COLOR AND PERFECTING EFFECT SPF 25 (BRONCE METALLIC) - BROWN
octinoxate and zinc oxide lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.0392 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CERESIN (UNII: Q1LS2UJO3A) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) SHEA BUTTER (UNII: K49155WL9Y) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) CERAMIDE NP (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) DIMETHICONE (UNII: 92RU3N3Y1O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SOYBEAN OIL (UNII: 241ATL177A) TOCOPHEROL (UNII: R0ZB2556P8) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) MICA (UNII: V8A1AW0880) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) D&C RED NO. 21 (UNII: 08744Z6JNY) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/12/2016 Labeler - Ventura Corporation LTD DUNS (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(13537-958, 13537-959, 13537-960, 13537-966, 13537-967, 13537-968, 13537-969, 13537-970, 13537-971, 13537-972, 13537-973, 13537-974, 13537-975, 13537-976, 13537-977, 13537-978, 13537-979, 13537-980, 13537-981, 13537-982, 13537-983, 13537-984, 13537-985, 13537-986, 13537-987)