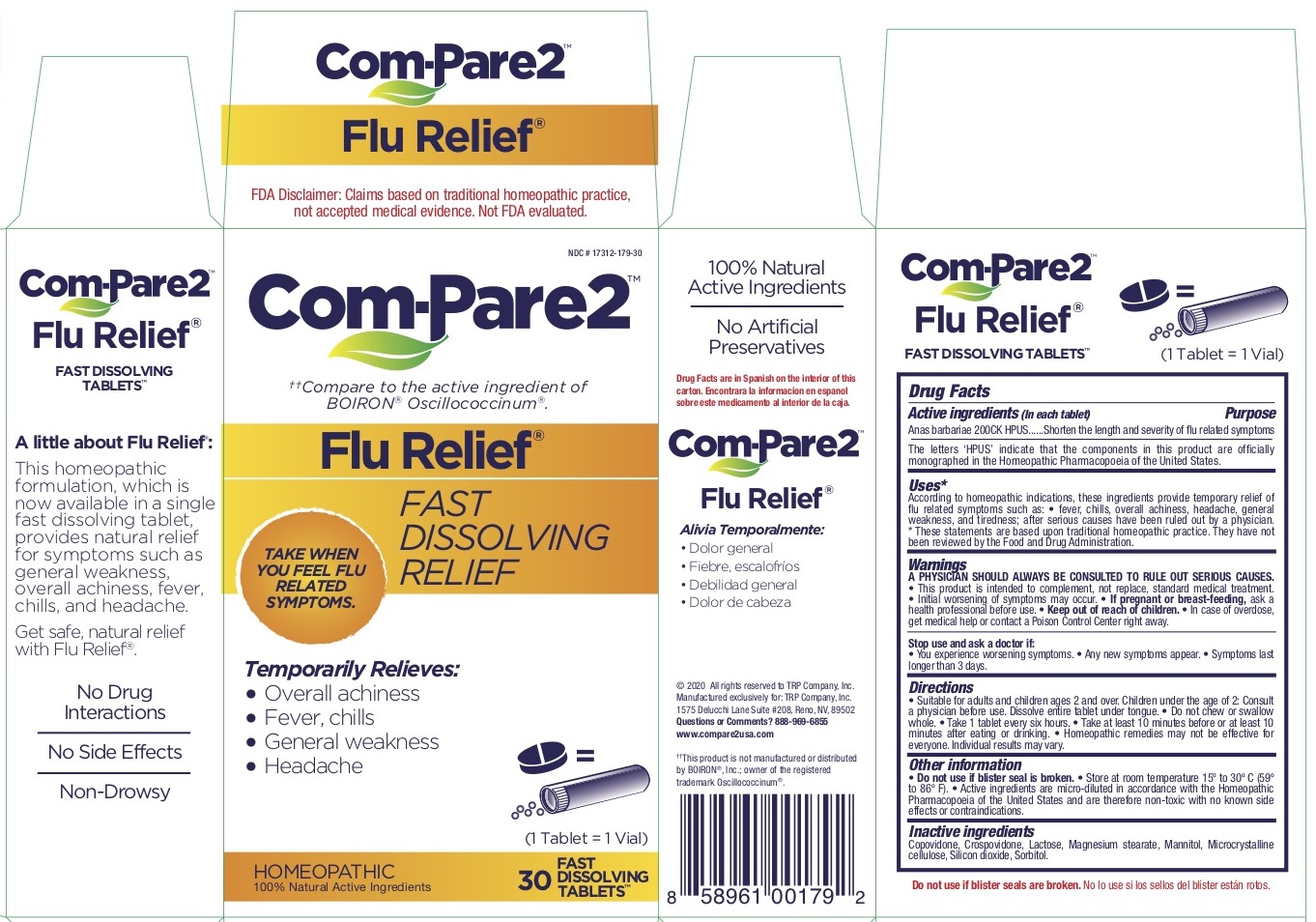
Label: COM-PARE2 FLU RELIEF- anas barbariae tablet
- NDC Code(s): 17312-179-30
- Packager: TRP Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Inactive Ingredients
-
Uses*
According to homeopathic indications, these ingredients provide temporary relief of
flu related symptoms such as: • fever, chills, overall achiness, headache, general
weakness, and tiredness; after serious causes have been ruled out by a physician.
* These statements are based upon traditional homeopathic practice. They have not
been reviewed by the Food and Drug Administration. -
Warnings
A PHYSICIAN SHOULD ALWAYS BE CONSULTED TO RULE OUT SERIOUS CAUSES.
• This product is intended to complement, not replace, standard medical treatment.
• Initial worsening of symptoms may occur. • If pregnant or breast-feeding, ask a
health professional before use. • Keep out of reach of children. • In case of overdose,
get medical help or contact a Poison Control Center right away. - Keep out of reach of children.
- If pregnant or breast-feeding.
- Stop use and ask a doctor if:
-
Directions
Directions
• Suitable for adults and children ages 2 and over. Children under the age of 2: Consult
a physician before use. Dissolve entire tablet under tongue. • Do not chew or swallow
whole. • Take 1 tablet every six hours. • Take at least 10 minutes before or at least 10
minutes after eating or drinking. • Homeopathic remedies may not be effective for
everyone. Individual results may vary. - Do not use
- Store at room temperature
- Other Information
- Label
-
INGREDIENTS AND APPEARANCE
COM-PARE2 FLU RELIEF
anas barbariae tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17312-179 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE (UNII: RN2HC612GY) (CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE - UNII:RN2HC612GY) CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE 200 [hp_C] Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) MANNITOL (UNII: 3OWL53L36A) CROSPOVIDONE (UNII: 2S7830E561) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SORBITOL (UNII: 506T60A25R) COPOVIDONE K25-31 (UNII: D9C330MD8B) Product Characteristics Color white Score no score Shape ROUND Size 3mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17312-179-30 2 in 1 BOX 02/15/2021 1 15 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/15/2021 Labeler - TRP Company (105185719)