Label: L-GLUTAMINE powder, for solution
- NDC Code(s): 70954-417-10, 70954-417-20
- Packager: ANI Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use L-GLUTAMINE ORAL POWDER safely and effectively. See full prescribing information for L-GLUTAMINE ORAL POWDER.
L-GLUTAMINE, oral powder
Initial U.S. Approval: 2017
INDICATIONS AND USAGE
L-glutamine is an amino acid indicated to reduce the acute complications of sickle cell disease in adult and pediatric patients 5 years of age and older. (1) (1)
DOSAGE AND ADMINISTRATION
- 5 grams to 15 grams orally, twice daily based on body weight. (2)
- Each dose of L-glutamine should be mixed in 8 oz. (240 mL) of cold or room temperature beverage or 4 oz. to 6 oz. of food before ingestion. (2)
DOSAGE FORMS AND STRENGTHS
Oral Powder: 5 grams of L-glutamine powder per foil-plastic laminate packet. (3) (3)
CONTRAINDICATIONS
None (4) (4)
ADVERSE REACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS & USAGE
2 DOSAGE & ADMINISTRATION
2.1 Dosage
2.2 Preparation of Product
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS & USAGE
-
2 DOSAGE & ADMINISTRATION
2.1 Dosage
Administer L-glutamine orally, twice per day at the dose based on body weight according to Table 1.
Table 1. Recommended Dosing
Weight in kilograms
Weight in pounds
Per dose in grams
Per day in grams
Packets per dose
Packets per day
less than 30
less than 66
5
10
1
2
30 to 65
66 to 143
10
20
2
4
greater than 65
greater than 143
15
30
3
6
- 3 DOSAGE FORMS & STRENGTHS
- 4 CONTRAINDICATIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to L-glutamine in 187 patients, including 136 exposed for 6 months and 109 exposed for ≥1 year. L-glutamine was studied in 2 placebo-controlled clinical trials (a phase 3 study, n=230 and a phase 2 study, n=70). In these trials, patients with sickle cell anemia or sickle β0-thalassemia were randomized to receive L-glutamine (n=187) or placebo (n=111) orally twice daily for 48 weeks followed by 3 weeks of tapering. Both studies included pediatric and adult patients (5-58 years of age) and 54% were female. The majority of patients were black (97.3%), had a diagnosis of sickle cell anemia (89.9%) and were receiving hydroxyurea at baseline (63.4%).
Treatment discontinuation due to adverse reactions was reported in 2.7% (n=5) of patients receiving L-glutamine. These adverse reactions included one case each of hypersplenism, abdominal pain, dyspepsia, burning sensation, and hot flash.
Serious adverse reactions were reported in both treatment groups, more frequently in the placebo group, and were consistent with the underlying disease.
Three deaths (3/187=1.6%) occurred during the study in the L-glutamine treatment group as compared to none in the placebo treatment group. None of the deaths were considered to be related to L-glutamine treatment. Adverse reactions occurring in greater than 10% of patients treated with L-glutamine are shown in Table 2 below.
Table 2. Adverse Reactions Occurring at an Incidence > 10% in Clinical Studies of L-glutamine
Adverse reaction
L-glutamine
N = 187
(%)
Placebo
N = 111
(%)
Constipation
21
18
Nausea
19
14
Headache
18
15
Abdominal Pain*
17
16
Cough
16
14
Pain in extremity
13
7
Back pain
12
5
Chest pain
12
8
*Abdominal pain = abdominal pain and abdominal pain, upper
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on L-glutamine use in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. Animal reproduction studies were not conducted with L-glutamine.
Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. The background risk of major birth defects and miscarriage for the indicated population are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of L-glutamine in human milk, the effect on the breastfed infant or the effect on milk production. The developmental and health benefits from breastfeeding should be considered along with the mother's clinical need for L-glutamine and any potential adverse effects on the breastfed child from L-glutamine or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of L-glutamine have been established in pediatric patients 5 years and older. Use of L-glutamine is supported by evidence from 2 placebo-controlled studies in adult and pediatric patients with sickle cell disease. The clinical studies enrolled 110 pediatric patients in the following age groups: 46 children (5 years up to less than 12 years) and 64 adolescents (12 years to less than 17 years).
The safety and effectiveness of L-glutamine in pediatric patients with sickle cell disease younger than 5 years old has not been established.
8.5 Geriatric Use
Clinical studies of L-glutamine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- 10 OVERDOSAGE
-
11 DESCRIPTION
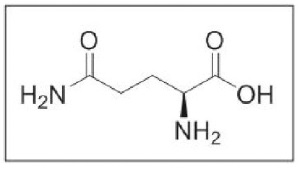
L-glutamine is an amino acid. L-glutamine is designated chemically as (S)-2-aminoglutaramic acid, L-glutamic acid 5-amide, or (S)-2,5-diamino-5-oxopentanoic acid. The molecular formula is C5H10N2O3 with the molecular weight of 146.15 g/mol and the following structural formula:
L-glutamine Oral Powder is formulated as a white to off-white colored powder and is packaged as 5 grams in a foil-plastic laminate packet for oral administration.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of the amino acid L-glutamine in treating sickle cell disease (SCD) is not fully understood. Oxidative stress phenomena are involved in the pathophysiology of SCD. Sickle red blood cells (RBCs) are more susceptible to oxidative damage than normal RBCs, which may contribute to the chronic hemolysis and vaso-occlusive events associated with SCD. The pyridine nucleotides, NAD+ and its reduced form NADH, play roles in regulating and preventing oxidative damage in RBCs. L-glutamine may improve the NAD redox potential in sickle RBCs through increasing the availability of reduced glutathione.
12.2 Pharmacodynamics
In vivo analyses demonstrated that L-glutamine supplementation improved NAD redox potential.
12.3 Pharmacokinetics
The pharmacokinetics of L-glutamine has been studied in healthy subjects and a variety of disease states. Relevant results from published literature are summarized below.
Absorption
Following single-dose oral administration of L-glutamine at 0.1 g/kg, mean peak L-glutamine concentration was 1028 μM (or 150 mcg/mL) occurring approximately 30 minutes after administration. The pharmacokinetics following multiple oral doses has not been characterized.
Distribution
After an intravenous (IV) bolus dose, the volume of distribution was estimated to be approximately 200 mL/kg.
Elimination
After an intravenous bolus dose, the terminal half-life of L-glutamine was approximately one hour.
Metabolism
Endogenous L-glutamine participates in various metabolic activities, including the formation of glutamate, and synthesis of proteins, nucleotides, and amino sugars. Exogenous L-glutamine is anticipated to undergo similar metabolism.
Excretion
Metabolism is the major route of elimination for L-glutamine. Although L-glutamine is eliminated by glomerular filtration, it is almost completely reabsorbed by the renal tubules.
Specific Populations
The safety of L-glutamine has not been established in patients with renal or hepatic impairment.
Drug Interactions
No drug interaction studies have been conducted.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of L-glutamine.
L-glutamine was not mutagenic in a bacterial mutagenicity (Ames) assay, nor clastogenic in a chromosome aberration assay in mammalian (Chinese Hamster Lung CHL/IU) cells.
Animal reproduction studies and its potential for impairment of fertility have not been conducted with L-glutamine. It is also not known whether L-glutamine can cause fetal harm when administered to a pregnant woman or whether it can affect reproductive capacity.
-
14 CLINICAL STUDIES
The efficacy of L-glutamine in sickle cell disease was evaluated in a randomized, double-blind, placebo-controlled, multi-center clinical trial entitled "A Phase III Safety and Efficacy Study of L-Glutamine to Treat Sickle Cell Disease or Sickle β0-thalassemia" [NCT01179217] (see Table 3).
The clinical trial evaluated the efficacy and safety of L-glutamine in 230 patients (5 to 58 years of age) with sickle cell anemia or sickle β0-thalassemia who had 2 or more painful crises within 12 months prior to enrollment. Eligible patients stabilized on hydroxyurea for at least 3 months continued their therapy throughout the study. The trial excluded patients who had received blood products within 3 weeks, had renal insufficiency or uncontrolled liver disease, or were pregnant (or planning pregnancy) or lactating. Study patients received L-glutamine or placebo for a treatment duration of 48 weeks followed by 3 weeks of tapering.
Efficacy was demonstrated by a reduction in the number of sickle cell crises through Week 48 and prior to the start of tapering among patients that received L-glutamine compared to patients who received placebo. This clinical benefit was observed irrespective of hydroxyurea use. A sickle cell crisis was defined as a visit to an emergency room/medical facility for sickle cell disease-related pain which was treated with a parenterally administered narcotic or parenterally administered ketorolac. In addition, the occurrence of chest syndrome, priapism, and splenic sequestration were considered sickle cell crises. Treatment with L-glutamine also resulted in fewer hospitalizations due to sickle cell pain at Week 48, fewer cumulative days in hospital and a lower incidence of acute chest syndrome.
Table 3. Results from the L-glutamine Clinical Trial in Sickle Cell Disease
Event
L-glutamine
(n = 152)
Placebo
(n = 78)
Median number of sickle cell crises (min, max)*
3 (0, 15)
4 (0, 15)
Median number of hospitalizations for sickle cell pain (min, max)*
2 (0, 14)
3 (0, 13)
Median cumulative days hospitalized (min, max)*
6.5 (0, 94)
11 (0, 187)
Median time (days) to first sickle cell crisis (95% CI)*,†
84 (62, 109)
54 (31, 73)
Patients with occurrences of acute chest syndrome (%)*
13 (8.6%)
18 (23.1%)
*Measured through 48 weeks of treatment
†Hazard Ratio=0.69 (95% CI=0.52, 0.93), estimated based on unstratified Cox's proportional model. Median time and 95% CI were estimated based on the Kaplan Meier method.
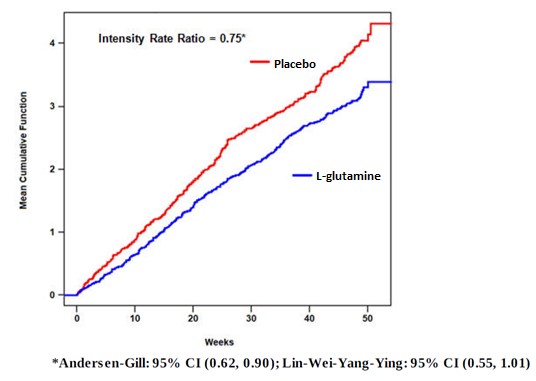
The recurrent crisis event time analysis (Figure 1) yielded an intensity rate ratio (IRR) value of 0.75 with 95% CI= (0.62, 0.90) and (0.55, 1.01) based on unstratified models using the Andersen-Gill and Lin, Wei, Yang and Ying methods, respectively in favor of L-glutamine, suggesting that over the entire 48-week period, the average cumulative crisis count was reduced by 25% from the L-glutamine group over the placebo group.
Figure 1. Recurrent Event Time for Sickle Cell Crises by Treatment Group
-
16 HOW SUPPLIED/STORAGE AND HANDLING
L-glutamine Oral Powder is supplied in foil-plastic laminate packets containing 5 grams of L-glutamine white to off-white colored powder.
- Carton of 60 packets: NDC 70954-417-20
Store at 20º to 25ºC (68º to 77ºF); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]; away from direct sunlight.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Dosage and Administration
Advise patient to take a missed dose as soon as they remember. Patient should not double the dose that they take.
Instruct patient to mix each dose in 8 oz. (240 mL) of cold or room temperature beverage or 4 to 6 oz. of food.
Advise patient that complete dissolution is not required prior to administration.
Manufactured by:
Novitium Pharma LLC
70 Lake Drive, East Windsor
New Jersey 08520
Issued: 02/2024
LB4394-01
-
SPL PATIENT PACKAGE INSERT
INSTRUCTIONS FOR USE
L-glutamine (l-gloo' ta meen)
Oral Powder
Read this Instructions for Use before you start taking L-glutamine and each time you get a refill. There may be new information. This Instructions for Use does not take the place of talking to your healthcare provider about your medical condition or treatment. You and your healthcare provider should talk about L-glutamine before you start taking it and at regular checkups.
L-glutamine is usually taken 2 times a day. Take L-glutamine as prescribed by your healthcare provider.
You will need the following supplies to mix and take
L-glutamine:
- Your prescribed dose of L-glutamine (1, 2, or 3 packets as directed by your healthcare provider).
- a clean cup or small bowl
- a spoon
You can mix L-glutamine:
- with a liquid, such as water, milk, or apple juice Or
- with food, such as applesauce or yogurt
How to mix and take a dose of L-glutamine.
Mixing with Liquid
Mixing with Food
Step 1:
Fill a cup with 8 ounces (240 mL) of liquid or a small bowl with 4 to 6 ounces of food.
The food or liquid should be cold or room temperature.
Do not use a hot food or liquid.


Step 2:
Tear open the L-glutamine packet at the top.


Step 3:
Pour the contents of the L-glutamine packet into the cup or bowl.
If more than 1 packet is needed, repeat steps 2 and 3 above for all of the packets needed to prepare your prescribed does of L-glutamine.
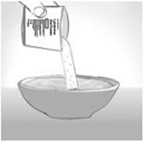
Step 4:
Use the spoon to mix the prescribed dose of L-glutamine with the liquid or food.
L-glutamine may not fully dissolve. You can take your dose of L-glutamine even if it does not fully dissolve.
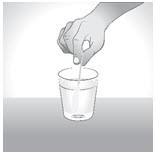
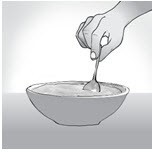
Step 5:
Drink or eat the prescribed dose of L-glutamine right away after mixing it.
Do not store the L-glutamine mixture for later use.
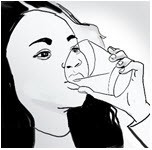
If you miss a dose of L-glutamine, take the missed dose as soon as you remember. Do not double the dose to make up for a missed dose.
How should I store L-glutamine?
- Store L-glutamine at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep L-glutamine away from direct sunlight.
Keep L-glutamine and all medicines out of the reach of children.
Manufactured by:
Novitium Pharma LLC
70 Lake Drive, East Windsor
New Jersey 08520
Issued: 02/2024
LB4394-01
For more information call 1-855-204-1431.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
L-GLUTAMINE
l-glutamine powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70954-417 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLUTAMINE (UNII: 0RH81L854J) (GLUTAMINE - UNII:0RH81L854J) GLUTAMINE 5 g Product Characteristics Color WHITE (white to off-white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70954-417-20 60 in 1 CARTON 07/11/2024 1 NDC:70954-417-10 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215647 07/11/2024 Labeler - ANI Pharmaceuticals, Inc. (145588013) Establishment Name Address ID/FEI Business Operations Novitium Pharma LLC 080301870 ANALYSIS(70954-417) , LABEL(70954-417) , MANUFACTURE(70954-417) , PACK(70954-417)




