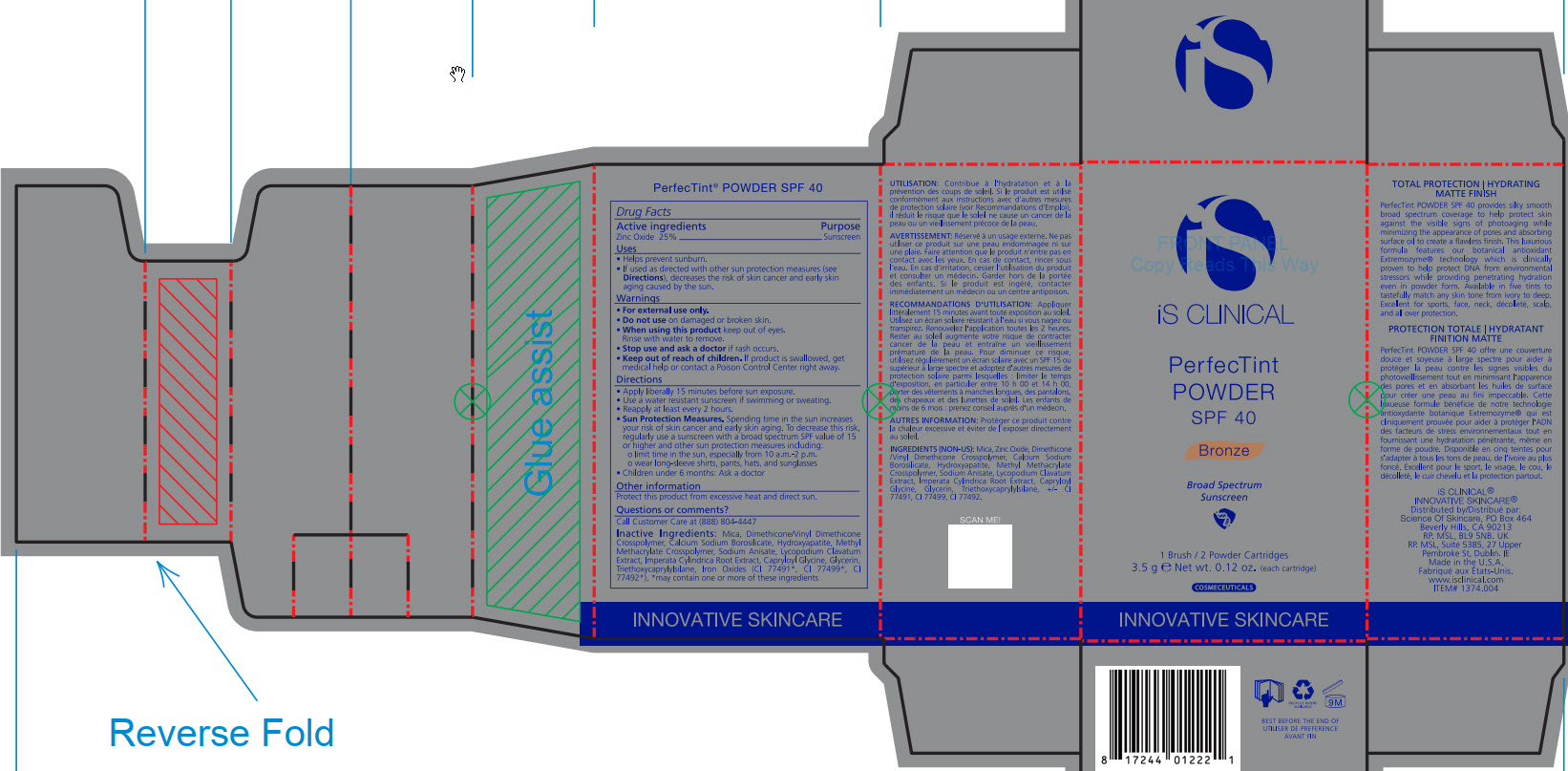
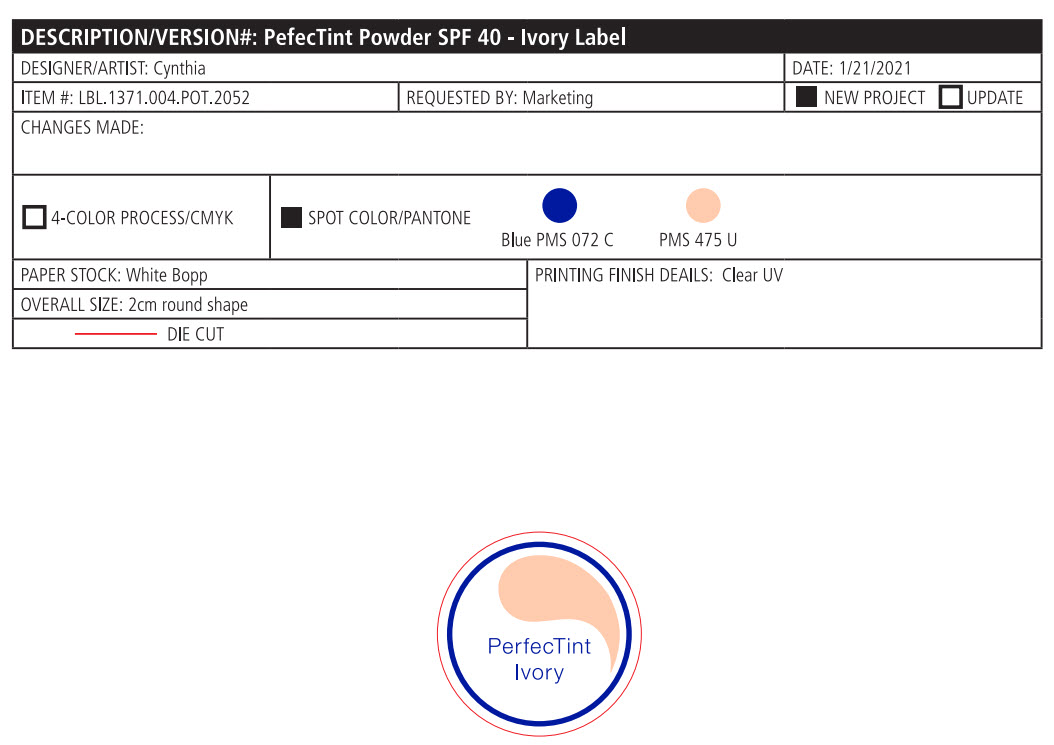
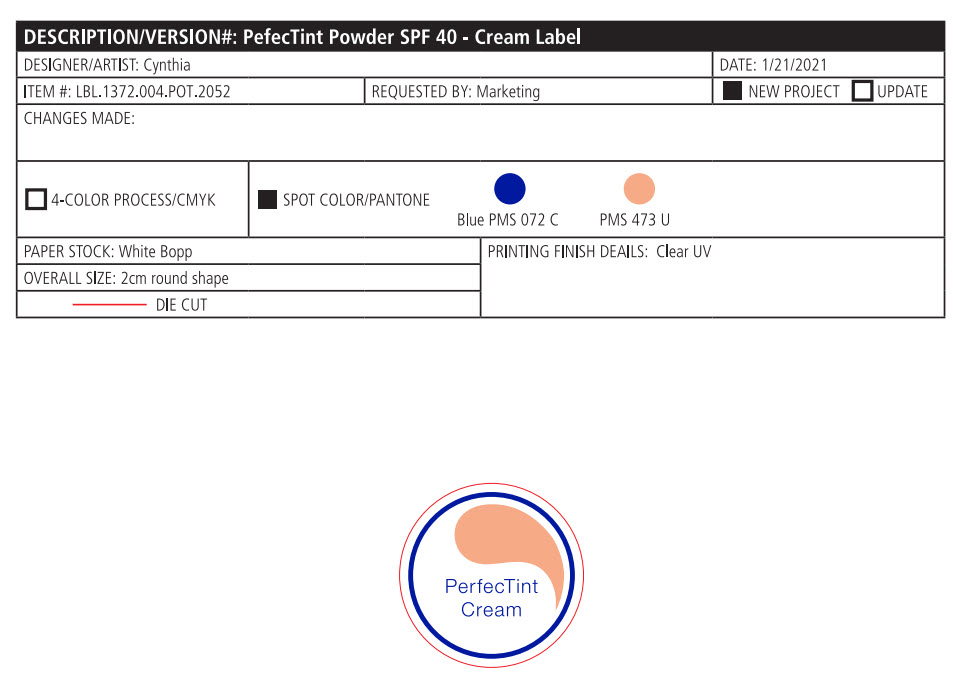
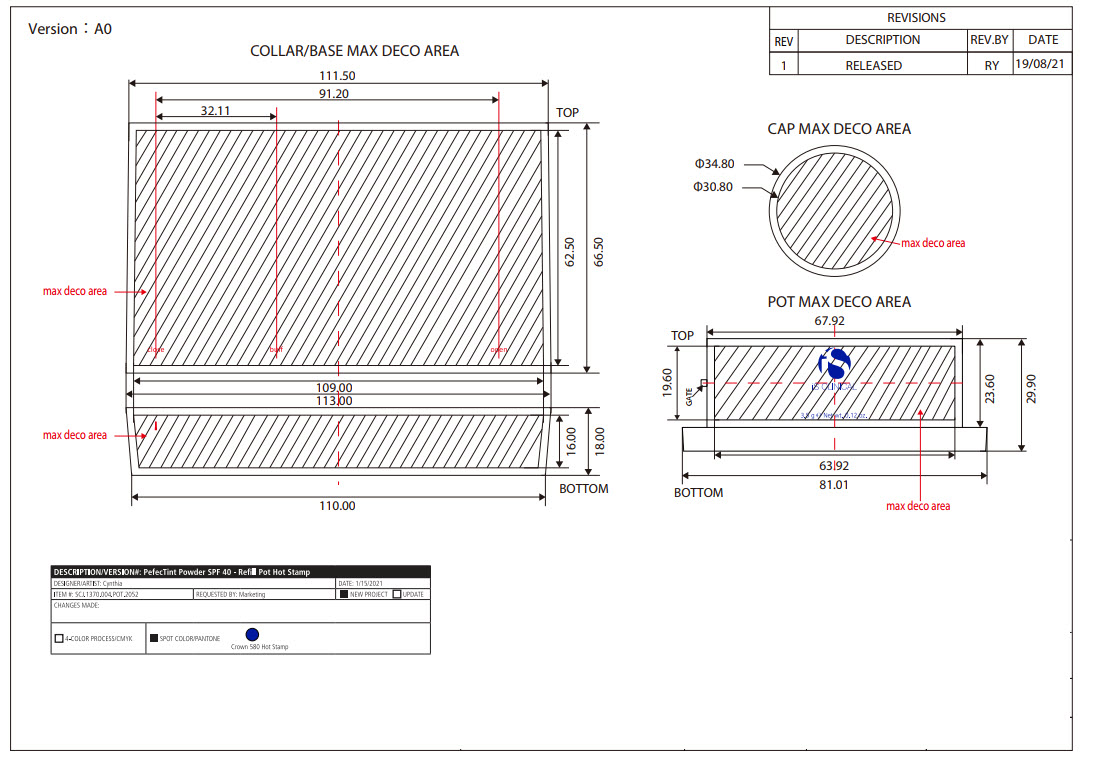
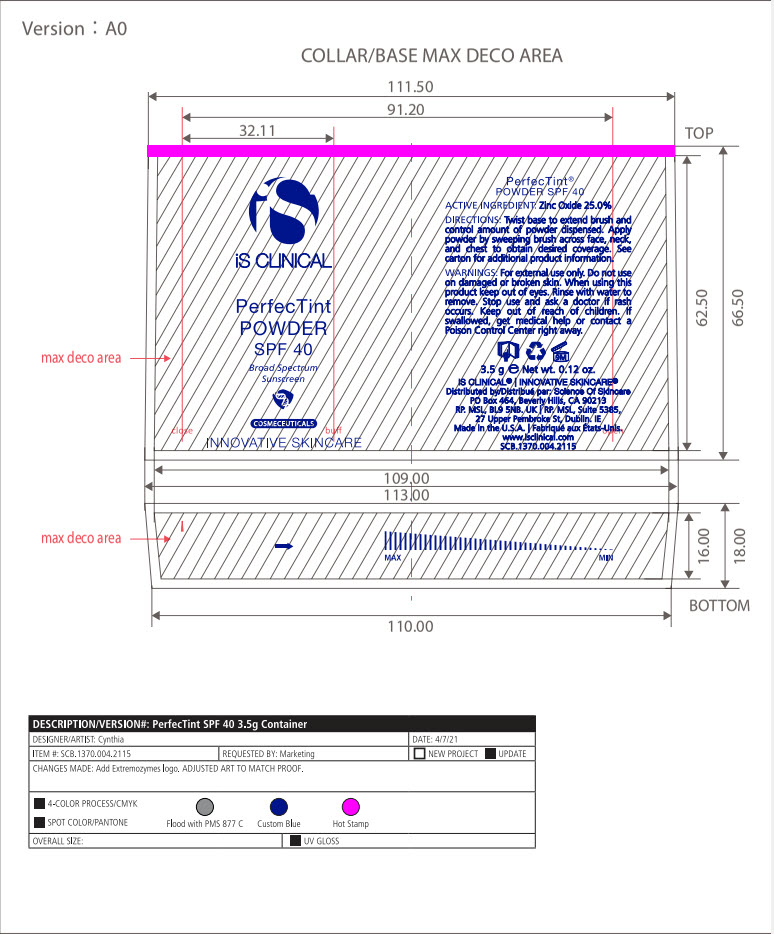
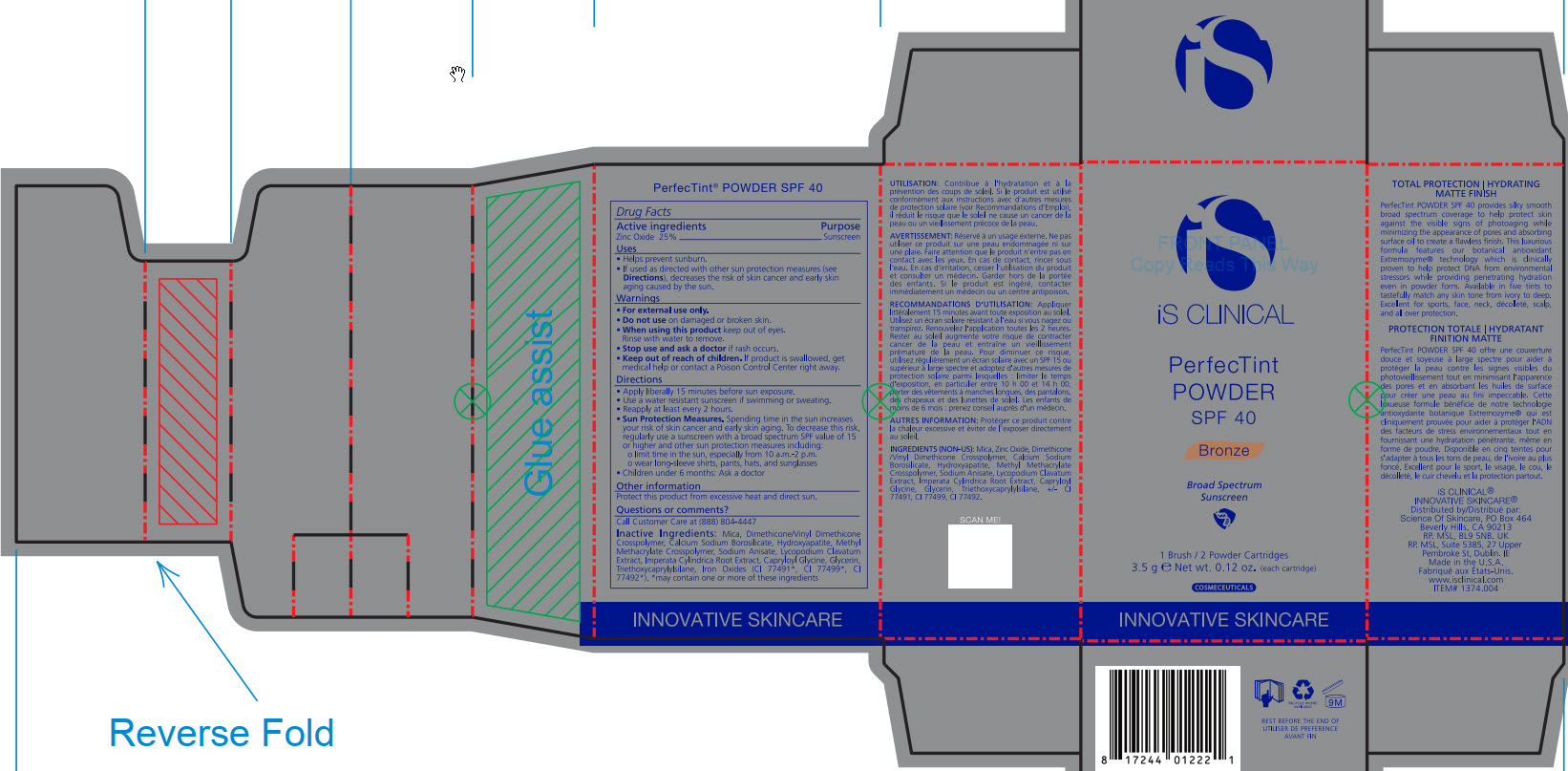
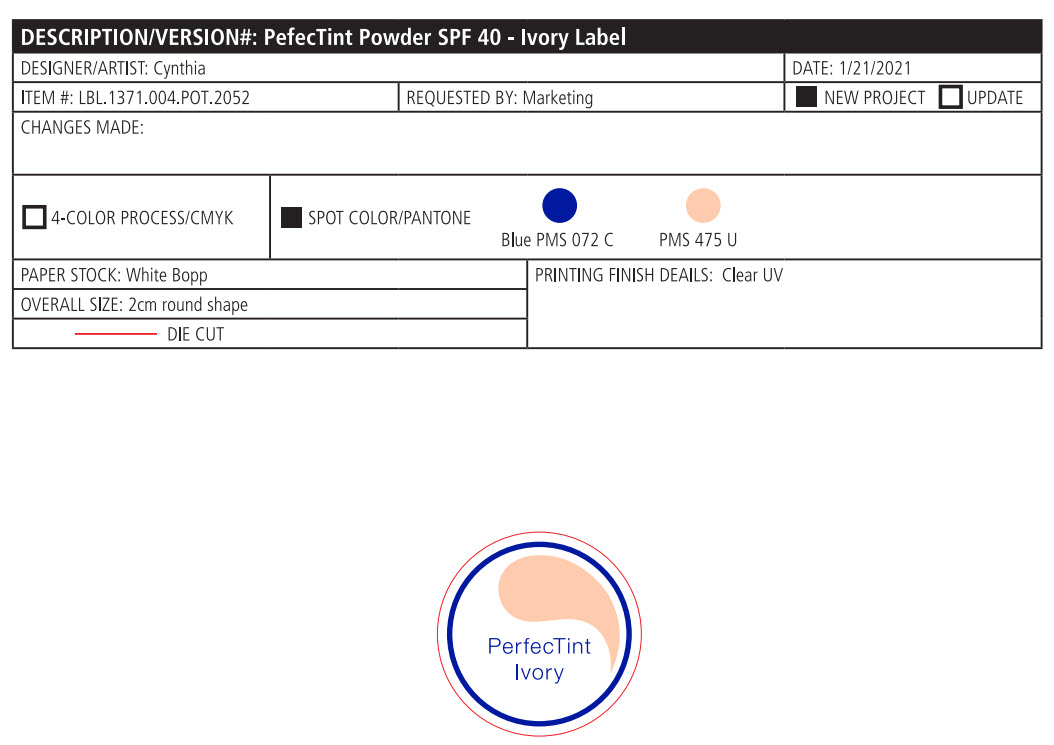
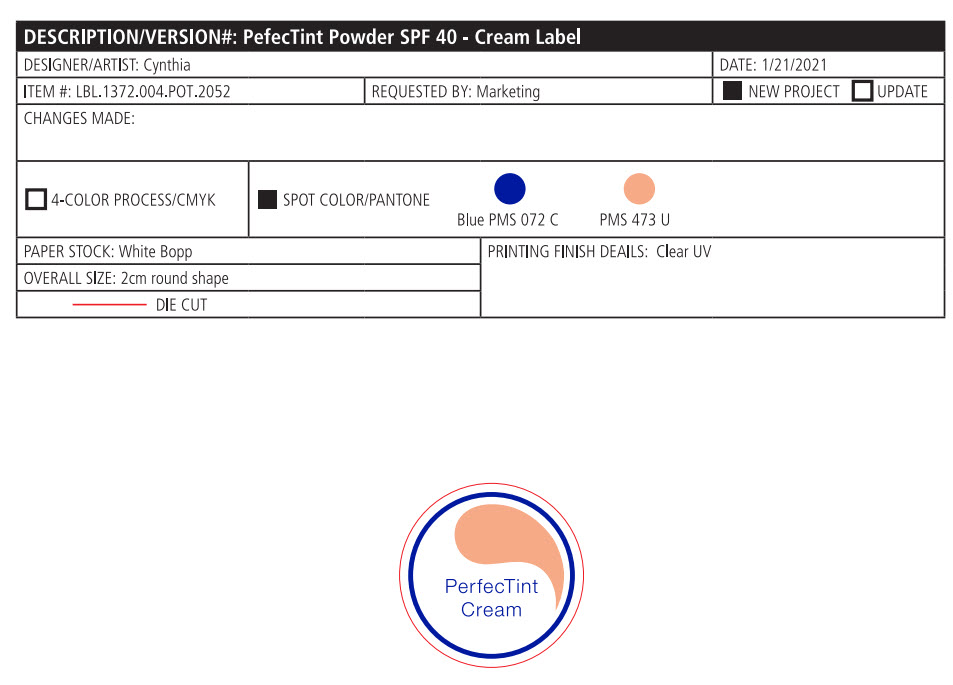
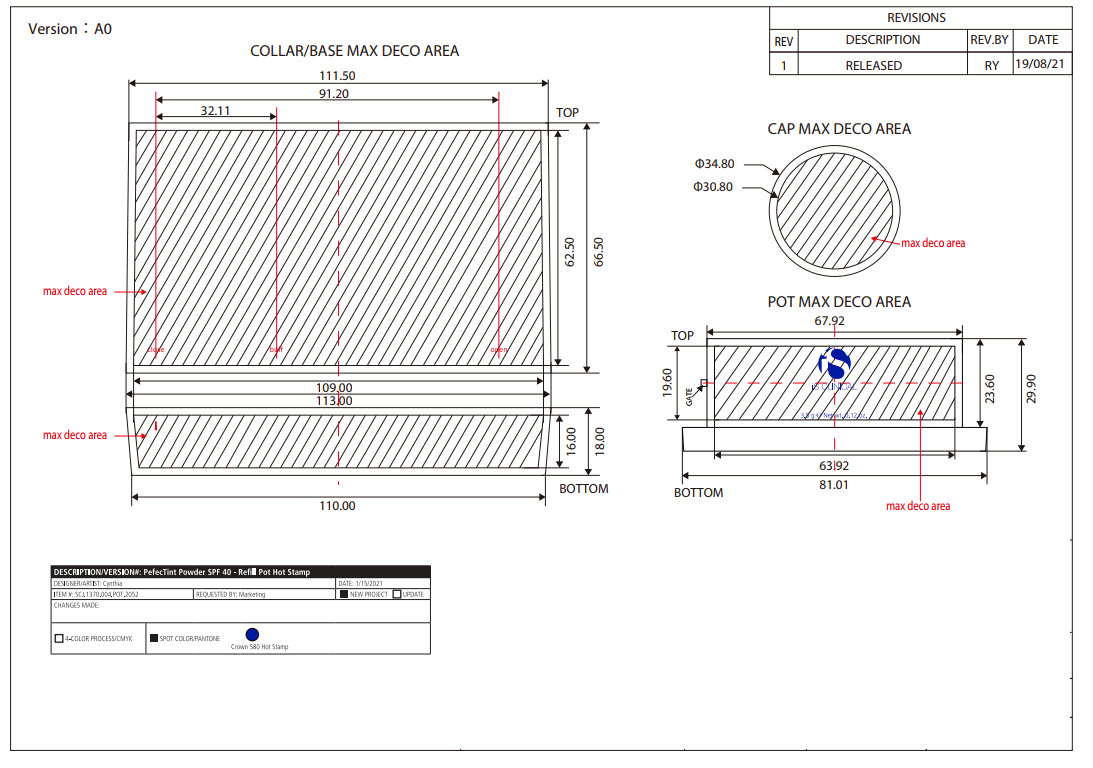
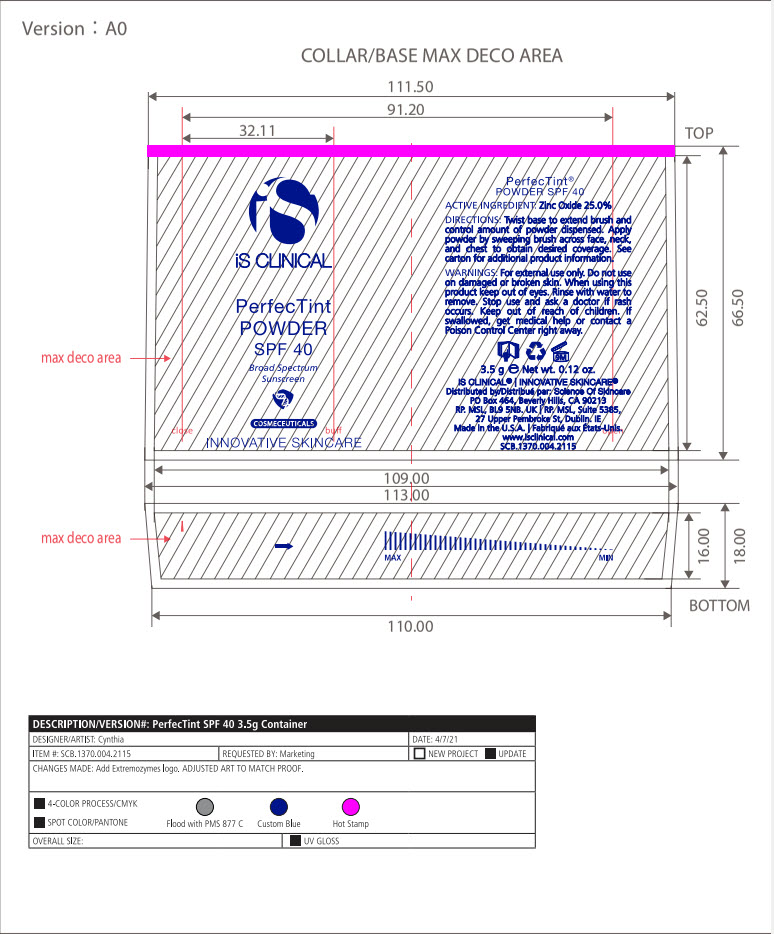
Label: PERFECTINT POWDER SPF 40 ALL SHADES- zinc oxide powder
- NDC Code(s): 69219-111-01, 69219-111-11
- Packager: Science of Skincare
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
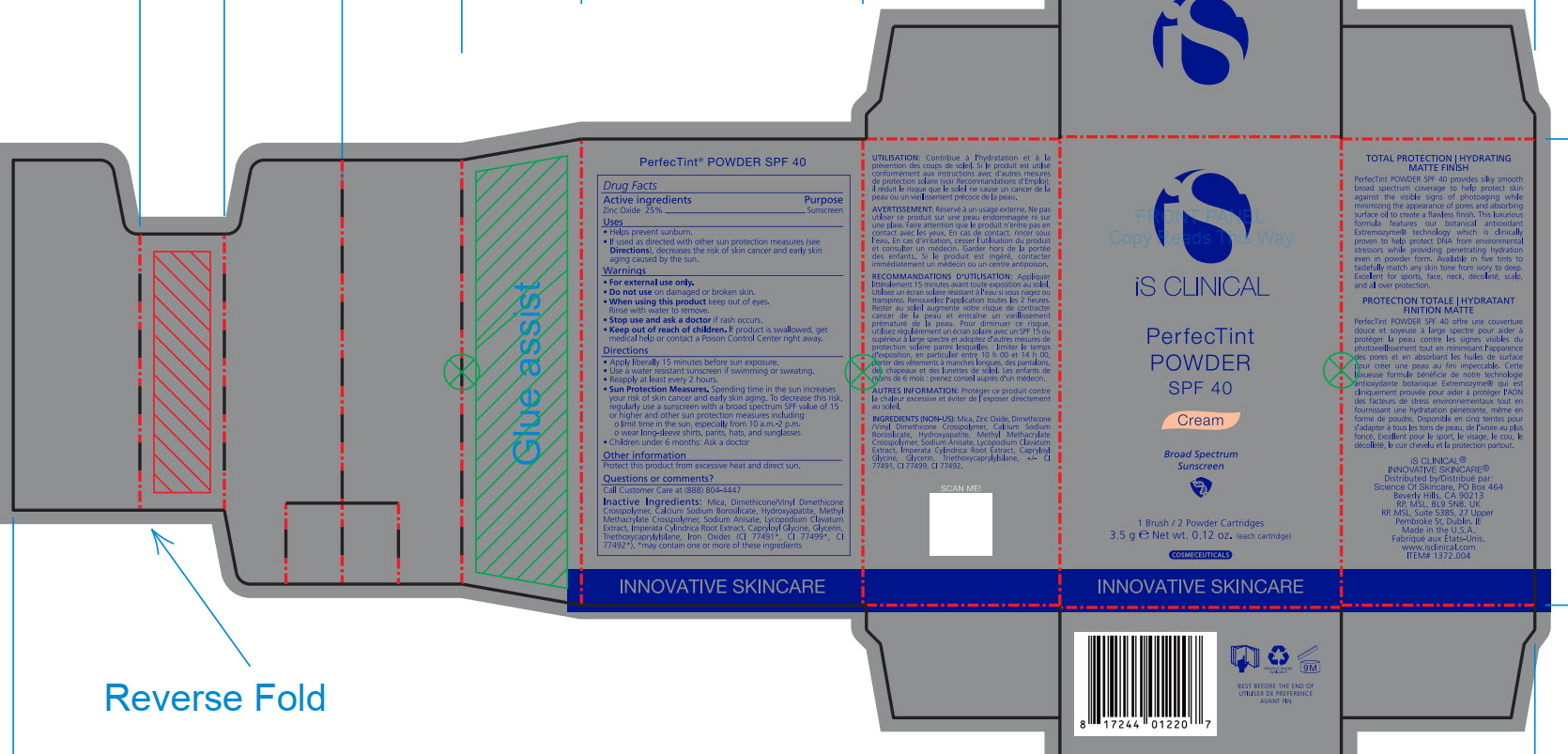
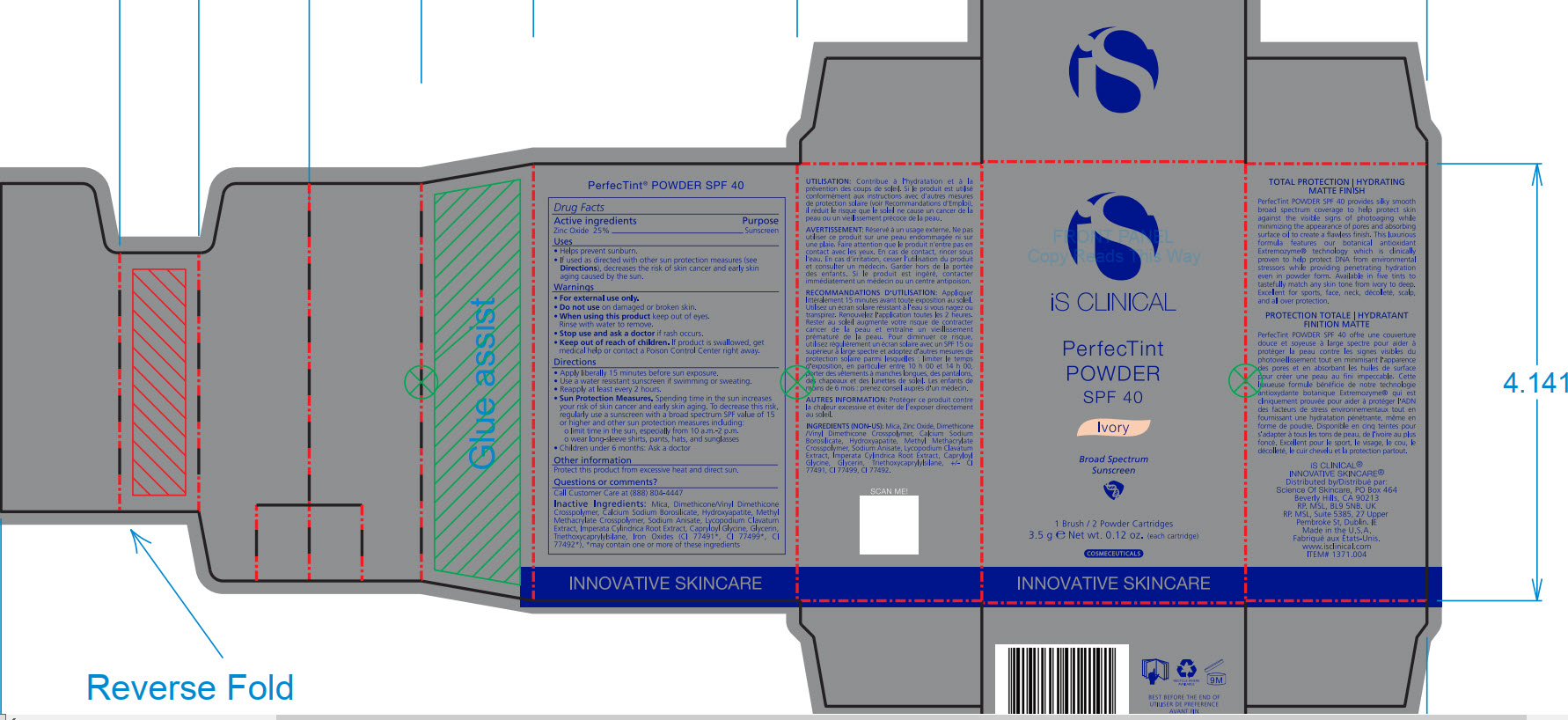
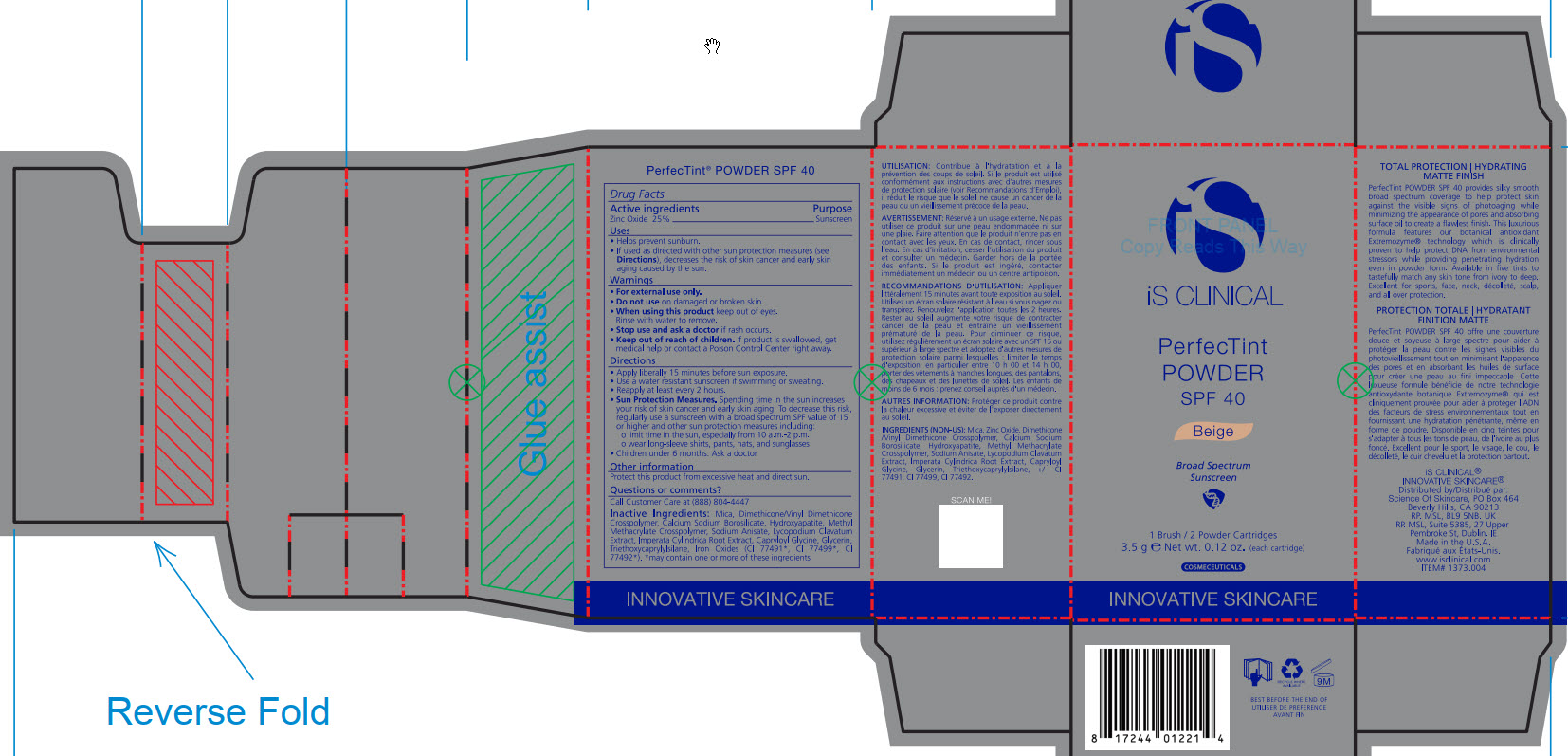
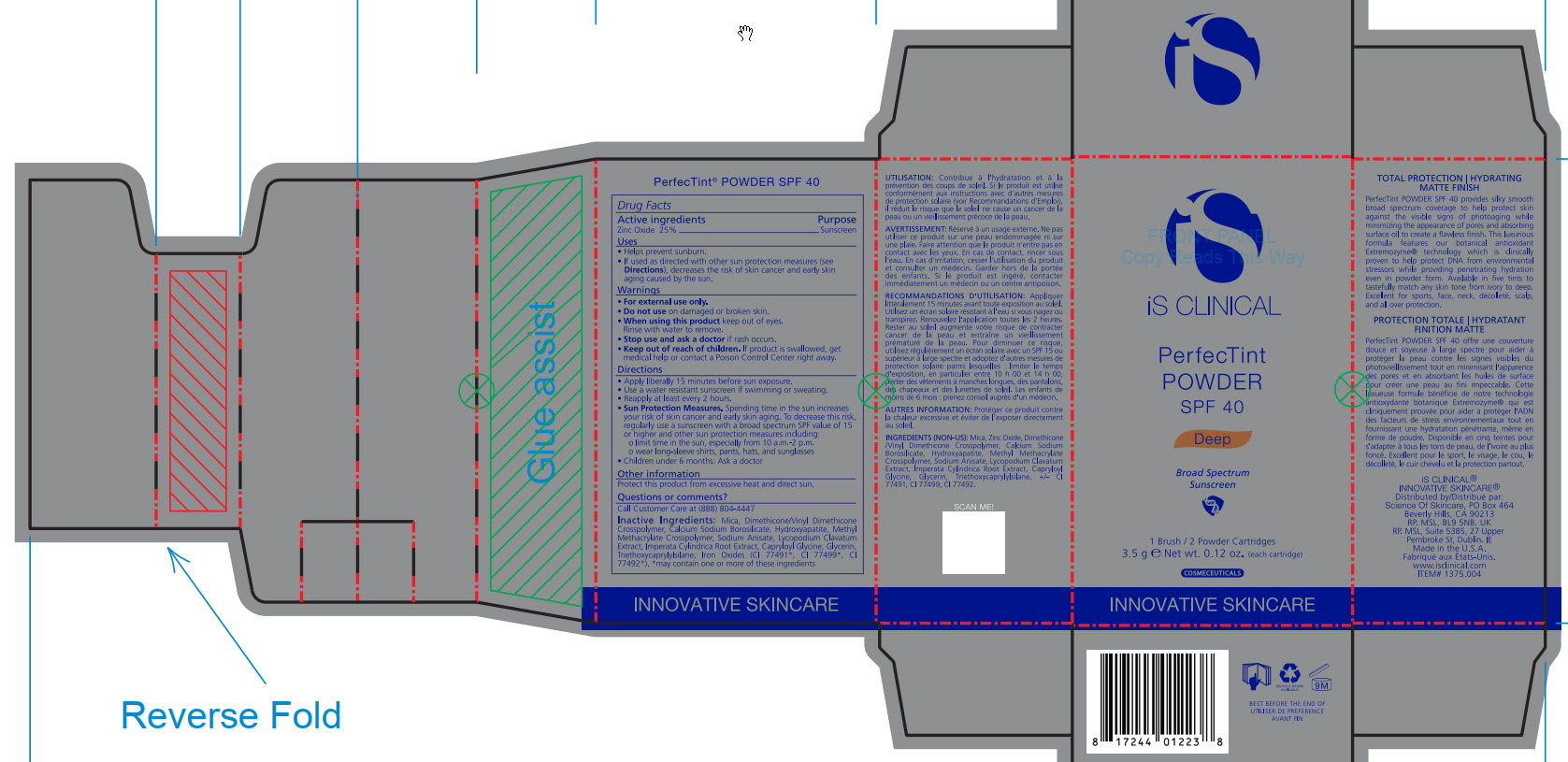
Apply liberally 15 minutes before sun exposure.
Use a water-resistant sunscreen if swimming or sweating.
Reapply at least every 2 hours.
Sun Protection Measures. Spending time in the sun increase your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:
limit time in the sun, especially from 10am - 2pm.
wear long-sleeve shirts, pants, hats, and sunglasses
Children under 6 months: Ask a doctor
- OTHER SAFETY INFORMATION
- QUESTIONS
-
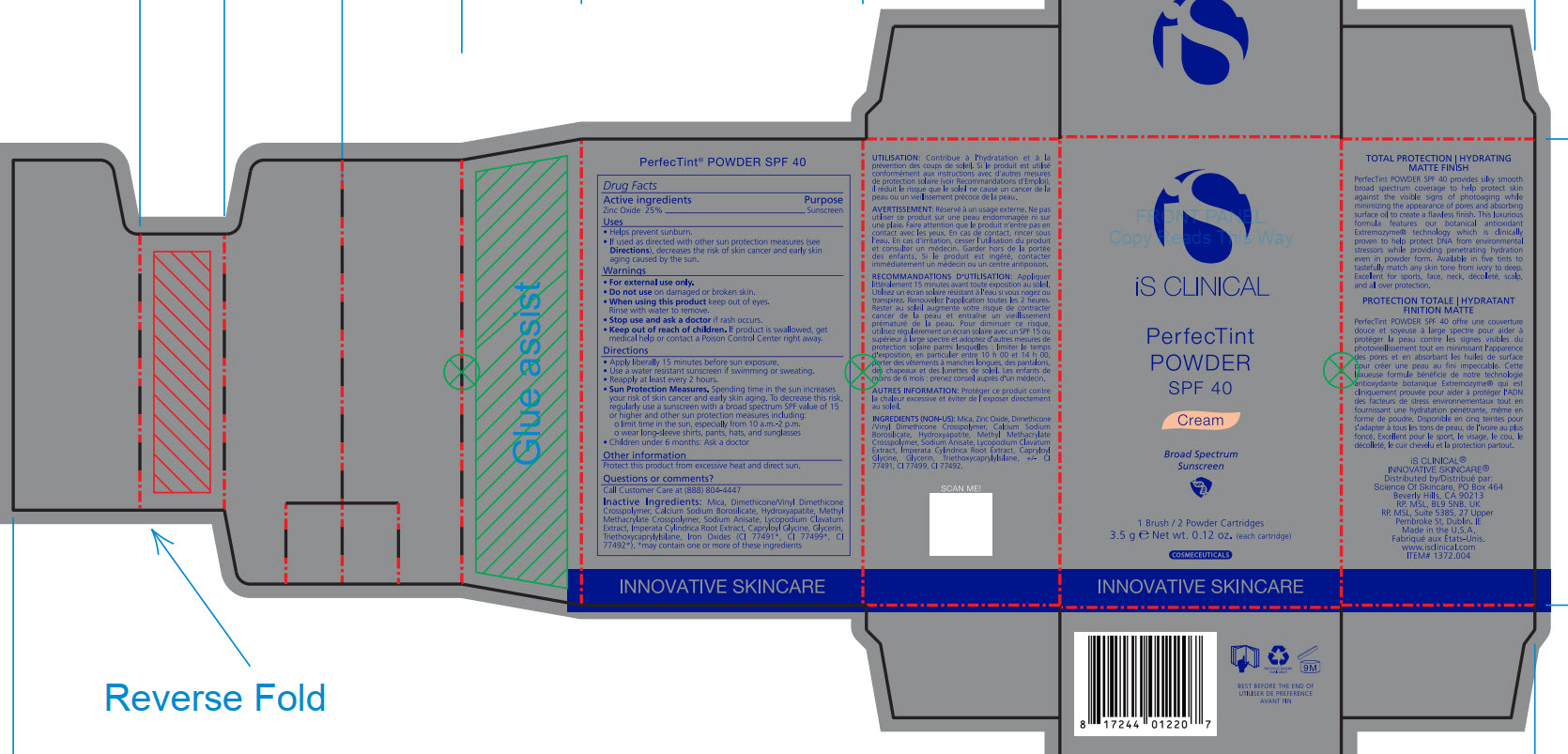
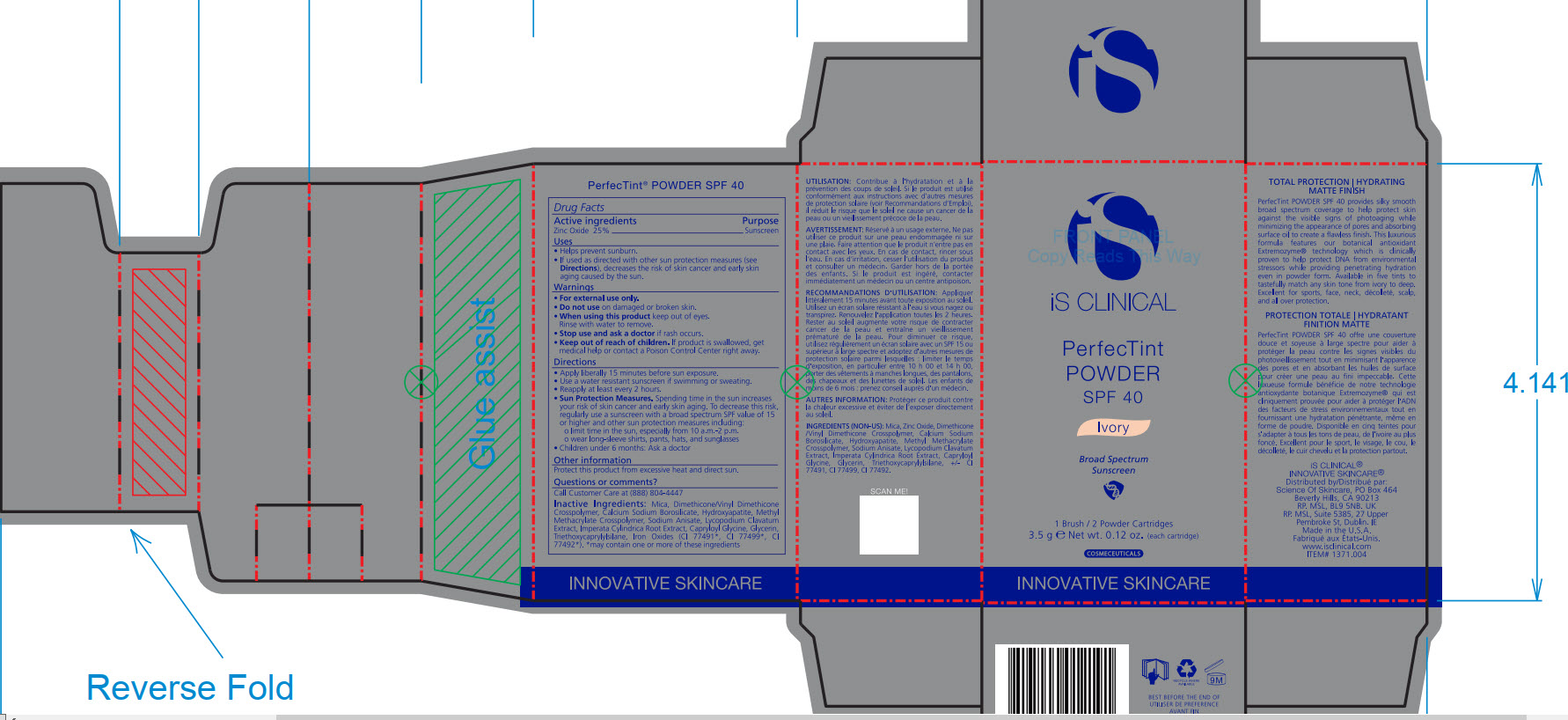
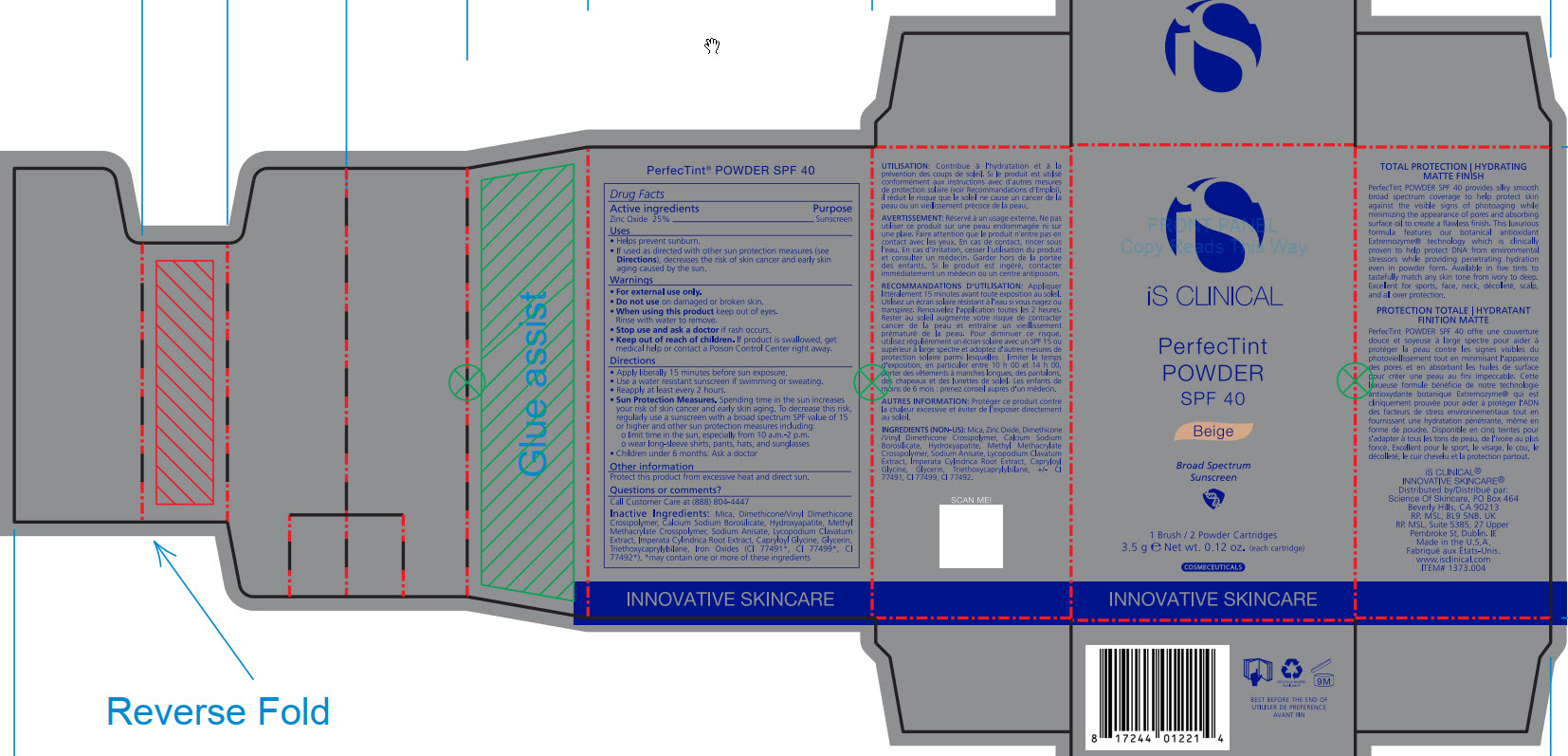
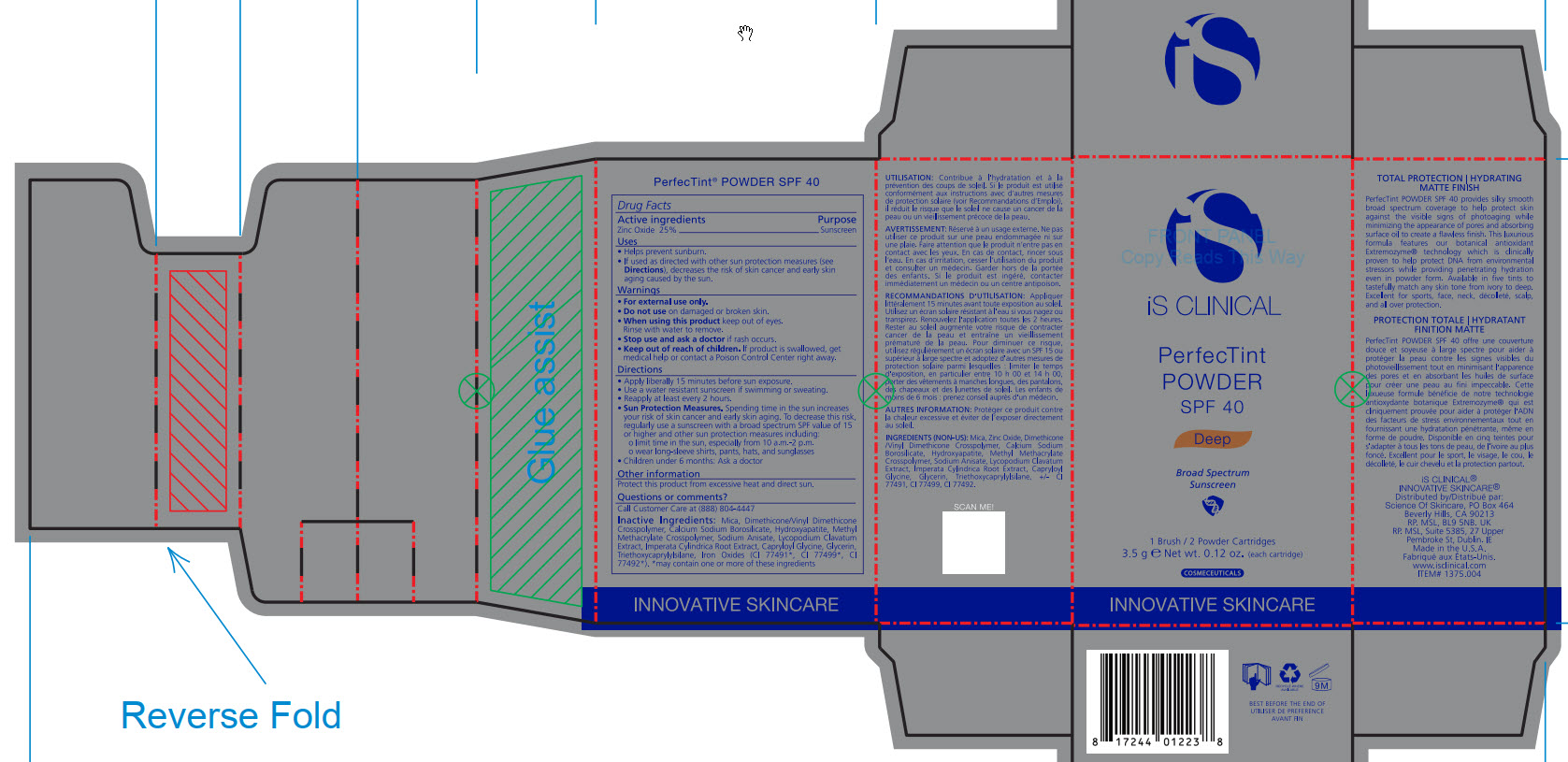
INACTIVE INGREDIENT
Mica, Dimethicone/Vinyl Dimethicone Crosspolymer, Calcium Sodium Borosilicate, Hydroxyapatite, Methyl Methacrylate Crosspolymer, Sodium Anisate, Lycopodium Clavatum Extract, Imperata Cylindrica Root Extract, Capryloyl Glycine, Glycerin, Triethoxycaprylylsiliane, Ironxe Oxides(CI 77491*, CI 77499*, CI77492*). *may contain one or more of these ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PERFECTINT POWDER SPF 40 ALL SHADES
zinc oxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69219-111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 25 g in 100 g Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) MICA (UNII: V8A1AW0880) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) SODIUM ANISATE (UNII: F9WFJ28MV9) LYCOPODIUM CLAVATUM WHOLE (UNII: 005ICF6L27) CAPRYLOYL GLYCINE (UNII: 8TY5YO42NJ) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) IMPERATA CYLINDRICA ROOT (UNII: VYT2JA85NH) GLYCERIN (UNII: PDC6A3C0OX) FERROSOFERRIC OXIDE (UNII: XM0M87F357) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69219-111-01 3.5 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/19/2023 2 NDC:69219-111-11 1 in 1 BOX 12/19/2023 2 NDC:69219-111-01 3.5 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/19/2023 Labeler - Science of Skincare (006251958)