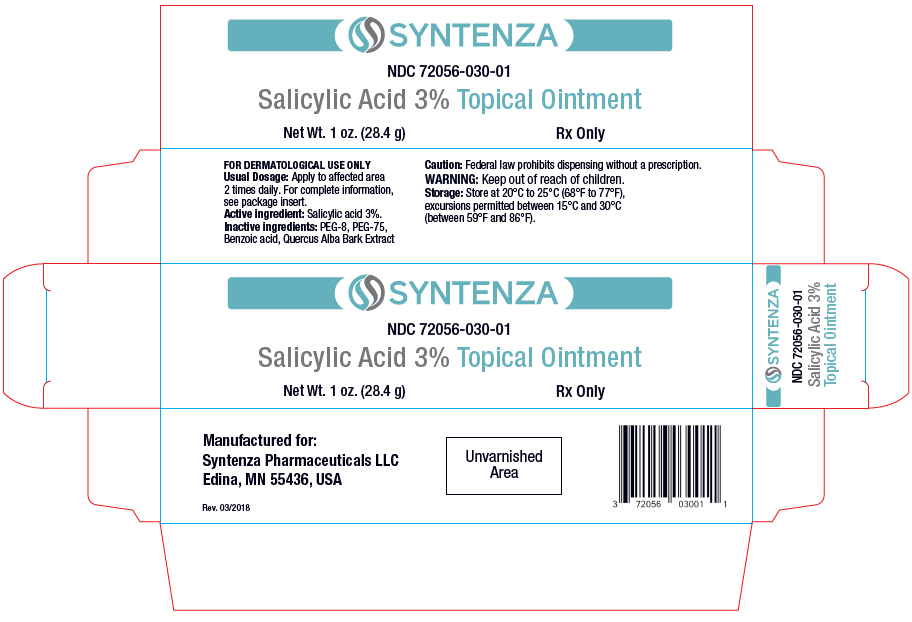
Label: SALICYLIC ACID ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 72056-030-01 - Packager: Syntenza Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 16, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
-
CLINICAL PHARMACOLOGY
The mechanism of action of Salicylic Acid 3% topical ointment is not known. While the following animal data are available, their clinical significance is unknown. It has been demonstrated that Salicylic Acid 3% topical ointment significantly reduces methicillin-resistant Staphylococcus aureus (MRSA) protected by biofilms in wounds using porcine models. In addition, Salicylic Acid 3% topical ointment stimulates re-epithelialization of second-degree burns in porcine models.
-
CLINICAL STUDIES
A randomized, double-blind, placebo-controlled study evaluated the rate of wound re-epithelialization. Four partial-thickness wounds (2×2 cm & 0.2 mm deep) were created under local anesthesia on the thighs of 13 normal, healthy, male volunteers with an electrokeratome. Salicylic Acid 3% topical ointment substantially increased the rate of re-epithelialization by 63% over the vehicle alone (p<0.01) and 77% over untreated control (p<0.005).
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- PRECAUTIONS
- DRUG INTERACTIONS
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
Patients should be advised to follow these step-by-step instructions for application of Salicylic Acid 3% topical ointment:
Hands should be washed thoroughly.
When using tubes, the tip of the tube should not come into contact with the area to be treated; the tube should be recapped tightly after each application.
If applying with a cotton-tipped applicator, which is recommended, use once and discard.
Salicylic Acid 3% topical ointment should be applied twice a day for best results.
Gently rinse the area to be treated with saline or water and then pat dry. Salicylic Acid 3% topical ointment can be applied directly to the wound or placed on dry gauze and then placed on the wound. Wet-Packs or Wet-To-Dry Dressings are not recommended since they will dilute the ointment and decrease its effectiveness. Salicylic Acid 3% topical ointment is designed to provide moisture to the wound.
Spread a generous quantity of Salicylic Acid 3% topical ointment evenly over the desired area to yield a thin continuous layer of approximately 1/8 of an inch of thickness. There may be a mild warming sensation, or slight burning, to the treated area for 3-5 minutes after application. If irritation occurs or symptoms persist after 10 days, discontinue use and consult your physician.
Try to keep the area being treated clean and exposed to air when possible. Apply an appropriate dressing to shield the area from clothes or exposure to water or dirt.
If there is no improvement in the wound within 7 days, consult your physician for further evaluation of the wound. If there is no response to the ointment at all, then the wound should be re-evaluated for other contributing factors to the wound healing process.
- PEDIATRIC USE
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 28.4 g Tube Carton
-
INGREDIENTS AND APPEARANCE
SALICYLIC ACID
salicylic acid ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72056-030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 1 g Inactive Ingredients Ingredient Name Strength Benzoic Acid (UNII: 8SKN0B0MIM) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72056-030-01 1 in 1 CARTON 10/05/2018 1 28.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 10/05/2018 Labeler - Syntenza Pharmaceuticals LLC (080999747)