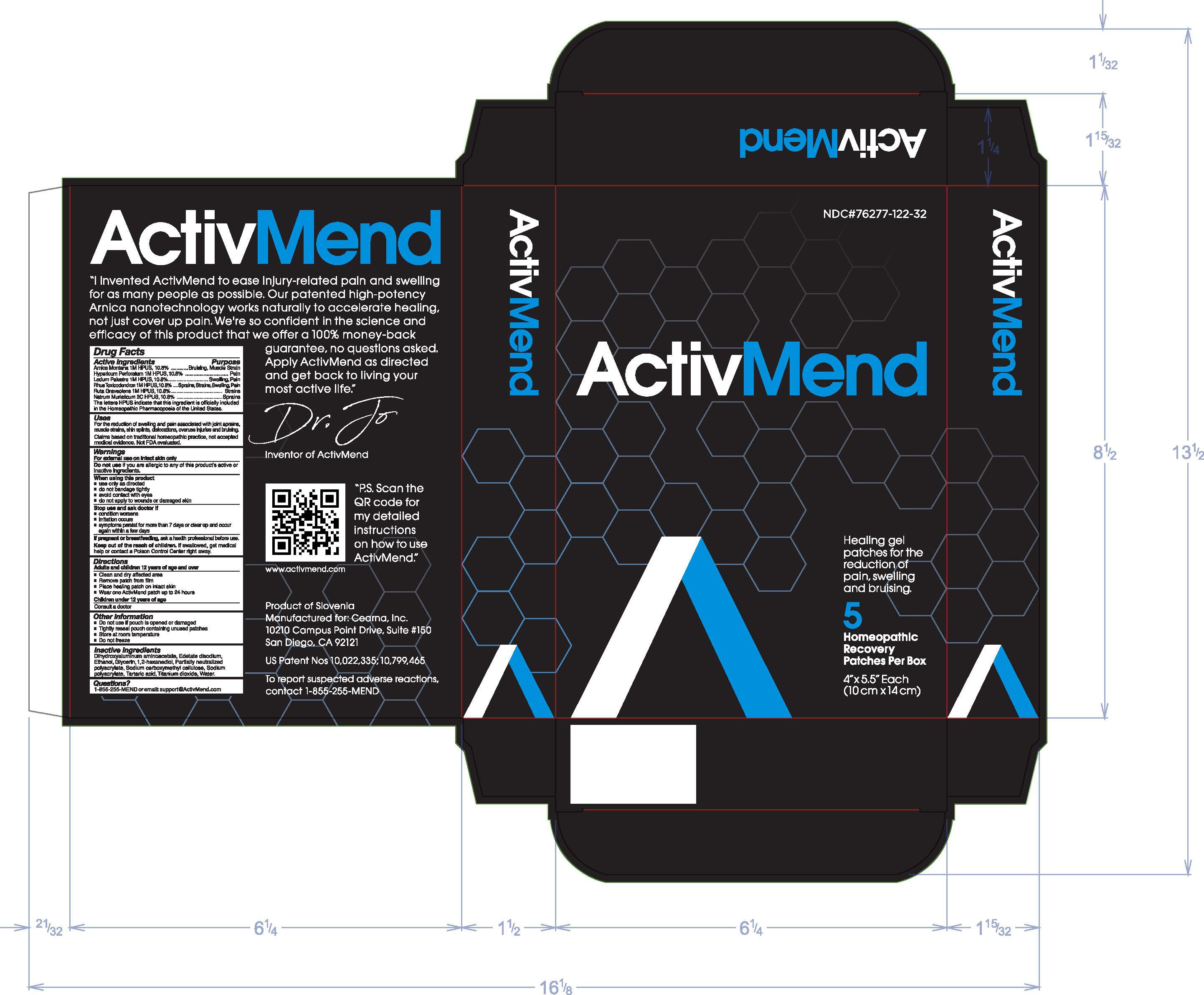
Label: ACTIVMEND- arnica montana, hypericum perforatum, ledum palustre, rhus toxicodendron, ruta graveolens, natrum muraticum patch
- NDC Code(s): 76277-122-32
- Packager: Cearna, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 8, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
-
Purpose
Arnica Montana 1M HPUS, 10.8%................Bruising, Muscle Strain
Hypericum Perforatum 1M HPUS, 10.8%.................Pain
Ledum Palustre 1M HPUS, 10.8%..............Swelling, Pain
Rhus Toxicodendron 1M HPUS, 10.8%................Sprains, Strains, Swelling, Pain
Ruta Graveolens 1M HPUS, 10.8%..............Strains
Natrum Muraticum 2C HPUS, 10.8%......................Sprains
The letter HPUS indicate that this ingredient is officially included in the Homeopathic Pharmacopoeia of the United States.
- Uses
-
Warnings
For external use on intact skin only
When using this product
- use only as directed
- do not bandage tightly
- avoid contact with eyes
- do not apply to wounds or damaged skin
- Directions
- Other information
- Inactive ingredients
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ACTIVMEND
arnica montana, hypericum perforatum, ledum palustre, rhus toxicodendron, ruta graveolens, natrum muraticum patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76277-122 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 1 [hp_M] in 1 g TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 1 [hp_M] in 1 g SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 2 [hp_C] in 1 g LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 1 [hp_M] in 1 g ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1 [hp_M] in 1 g RUTA GRAVEOLENS FLOWERING TOP (UNII: N94C2U587S) (RUTA GRAVEOLENS FLOWERING TOP - UNII:N94C2U587S) RUTA GRAVEOLENS FLOWERING TOP 1 [hp_M] in 1 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) ACRYLIC ACID/SODIUM ACRYLATE COPOLYMER (1:1; 600 MPA.S AT 0.2%) (UNII: M4PPW69Y4H) TARTARIC ACID (UNII: W4888I119H) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) GLYCERIN (UNII: PDC6A3C0OX) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76277-122-32 1 in 1 BOX 07/08/2021 1 5 in 1 POUCH 1 14 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/09/2021 Labeler - Cearna, Inc. (968104609) Registrant - Cearna, Inc. (968104609) Establishment Name Address ID/FEI Business Operations Wooshin Lapache d.o.o. 507385209 manufacture(76277-122)