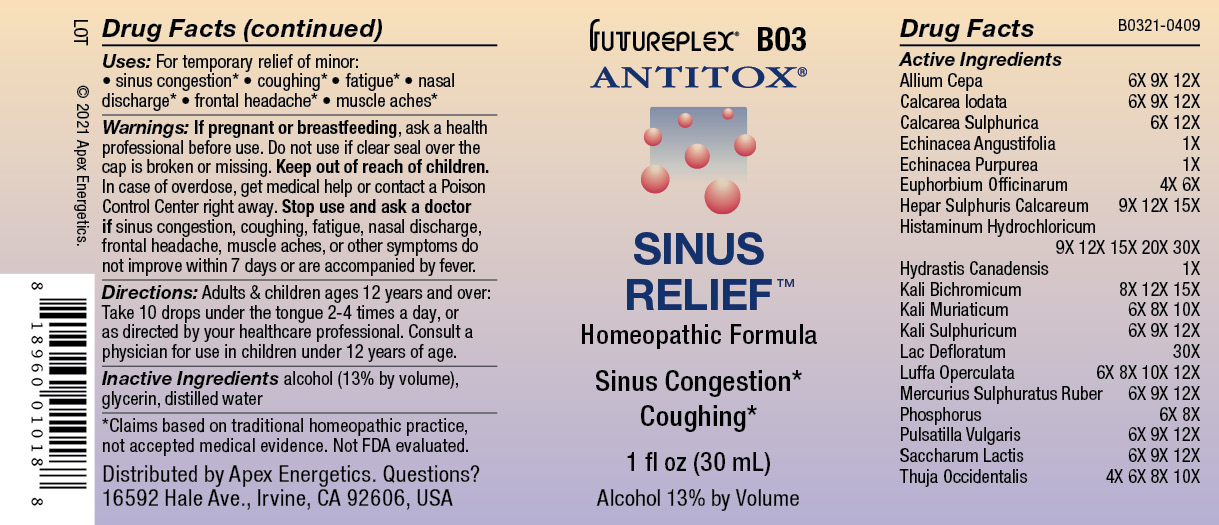
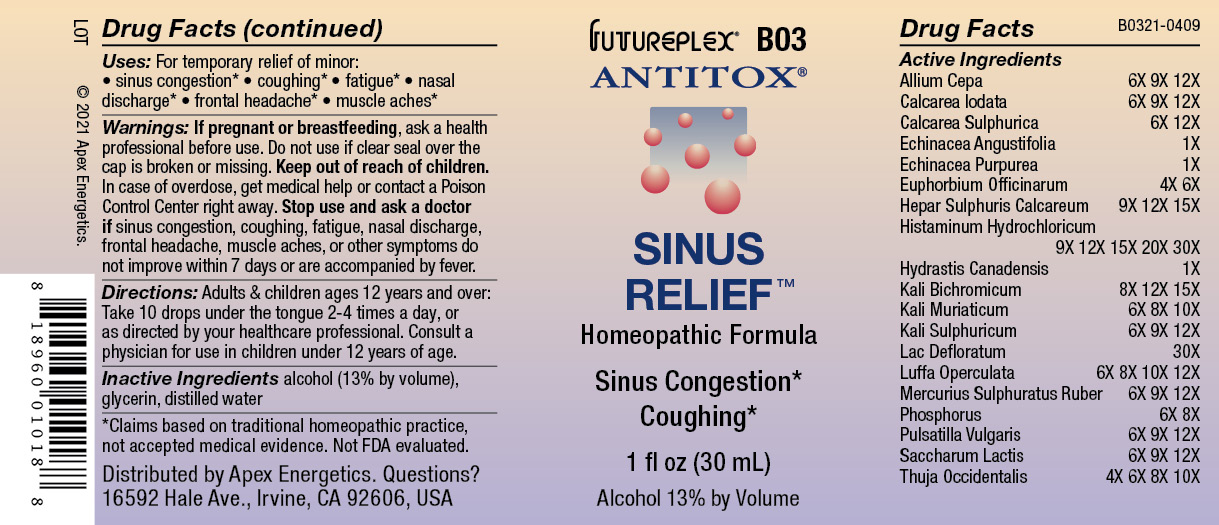
Label: B03 SINUS RELIEF- allium cepa, calcarea iodata, calcarea sulphurica, echinacea angustifolia, echinacea purpurea, euphorbium officinarum, hepar sulphuris calcareum, histaminum hydrochloricum, hydrastis canadensis, kali bichromicum, kali muriaticum, kali sulphuricum, lac defloratum, luffa operculata, mercurius sulphuratus ruber, phosphorus, pulsatilla vulgaris, saccharum lactis, thuja occidentalis solution/ drops
- NDC Code(s): 63479-0203-1
- Packager: Apex Energetics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients
Allium Cepa
6X 9X 12X
Calcarea Iodata
6X 9X 12X
Calcarea Sulphurica
6X 12X
Echinacea Angustifolia
1X
Echinacea Purpurea
1X
Euphorbium Officinarum
4X 6X
Hepar Sulphuris Calcareum
9X 12X 15X
Histaminum Hydrochloricum
9X 12X 15X 20X 30X
Hydrastis Canadensis
1X
Kali Bichromicum
8X 12X 15X
Kali Muriaticum
6X 8X 10X
Kali Sulphuricum
6X 9X 12X
Lac Defloratum
30X
Luffa Operculata
6X 8X 10X 12X
Mercurius Sulphuratus Ruber
6X 9X 12X
Phosphorus
6X 8X
Pulsatilla Vulgaris
6X 9X 12X
Saccharum Lactis
6X 9X 12X
Thuja Occidentalis
4X 6X 8X 10X
- INDICATIONS & USAGE
- Warnings:
- Directions:
- Inactive Ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
B03 SINUS RELIEF
allium cepa, calcarea iodata, calcarea sulphurica, echinacea angustifolia, echinacea purpurea, euphorbium officinarum, hepar sulphuris calcareum, histaminum hydrochloricum, hydrastis canadensis, kali bichromicum, kali muriaticum, kali sulphuricum, lac defloratum, luffa operculata, mercurius sulphuratus ruber, phosphorus, pulsatilla vulgaris, saccharum lactis, thuja occidentalis solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63479-0203 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) (ANHYDROUS LACTOSE - UNII:3SY5LH9PMK) LACTOSE, UNSPECIFIED FORM 12 [hp_X] in 1 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 10 [hp_X] in 1 mL POTASSIUM SULFATE (UNII: 1K573LC5TV) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM SULFATE 12 [hp_X] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 1 [hp_X] in 1 mL POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 15 [hp_X] in 1 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 10 [hp_X] in 1 mL PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 12 [hp_X] in 1 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 8 [hp_X] in 1 mL SKIM MILK (UNII: 6A001Y4M5A) (SKIM MILK - UNII:6A001Y4M5A) SKIM MILK 30 [hp_X] in 1 mL CALCIUM IODIDE (UNII: 8EKI9QEE2H) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM IODIDE 12 [hp_X] in 1 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA WHOLE - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 1 [hp_X] in 1 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA WHOLE - UNII:QI7G114Y98) ECHINACEA PURPUREA 1 [hp_X] in 1 mL EUPHORBIA RESINIFERA RESIN (UNII: 1TI1O9028K) (EUPHORBIA RESINIFERA RESIN - UNII:1TI1O9028K) EUPHORBIA RESINIFERA RESIN 6 [hp_X] in 1 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 15 [hp_X] in 1 mL HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 30 [hp_X] in 1 mL MERCURIC SULFIDE (UNII: ZI0T668SF1) (MERCURIC CATION - UNII:ED30FJ8Y42) MERCURIC SULFIDE 12 [hp_X] in 1 mL ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 12 [hp_X] in 1 mL CALCIUM SULFATE ANHYDROUS (UNII: E934B3V59H) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM SULFATE ANHYDROUS 12 [hp_X] in 1 mL LUFFA OPERCULATA FRUIT (UNII: C4MO6809HU) (LUFFA OPERCULATA FRUIT - UNII:C4MO6809HU) LUFFA OPERCULATA FRUIT 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63479-0203-1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 02/15/1995 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/15/1995 Labeler - Apex Energetics Inc. (195816384)